Global Neurology Clinical Trials Market - Key Trends & Drivers Summarized
Why Are Neurology Clinical Trials Gaining Importance in Drug Development?
Neurology clinical trials are at the forefront of medical research, driving the discovery of novel therapies for a wide range of neurological disorders, including Alzheimer’ s disease, Parkinson’ s disease, multiple sclerosis, epilepsy, and stroke. Given the complexity and multifactorial nature of neurological conditions, clinical trials play a crucial role in validating new treatments, assessing disease-modifying therapies, and improving symptom management. The rapid expansion of precision medicine, gene therapy, and biologics in neurology has further amplified the need for robust clinical trials to evaluate their safety and efficacy. Traditional drug development approaches in neurology have often faced setbacks due to the blood-brain barrier, which limits drug delivery to the central nervous system (CNS). However, advances in nanotechnology, biomarker-based patient stratification, and targeted drug delivery systems are now enhancing the success rates of neurology trials. Additionally, the emergence of AI-driven clinical trial design and patient recruitment strategies is optimizing trial efficiency, reducing costs, and accelerating timelines. As the global burden of neurodegenerative and neurological disorders rises, neurology clinical trials remain essential in addressing unmet medical needs and shaping the future of neuroscience therapeutics.What Challenges Are Slowing the Progress of Neurology Clinical Trials?
Despite their growing significance, neurology clinical trials face several challenges that hinder their progress and success rates. One of the most persistent issues is the high failure rate, with many neurology drug candidates failing to demonstrate efficacy in late-stage trials, leading to significant financial losses for pharmaceutical companies. The complexity of neurological diseases, coupled with the difficulty of measuring cognitive and functional outcomes objectively, poses challenges in endpoint determination and trial design. Patient recruitment and retention remain major bottlenecks, as neurological trials often require long study durations, frequent assessments, and invasive procedures such as lumbar punctures or brain imaging, which can discourage participation. Additionally, the heterogeneity of neurological disorders makes it difficult to identify suitable patient populations, requiring advanced biomarker-driven stratification approaches. The regulatory landscape for neurology trials is also stringent, with agencies requiring extensive safety data due to the risks associated with CNS-targeted therapies. Ethical considerations, particularly in conditions such as Alzheimer’ s and ALS, further complicate trial execution, as cognitive decline can impact informed consent and patient compliance. Addressing these challenges requires innovative trial methodologies, improved recruitment strategies, and enhanced regulatory frameworks to facilitate faster and more effective neurology clinical trials.How Are Technological Innovations Transforming Neurology Clinical Trials?
The neurology clinical trials market is experiencing a technological revolution, with cutting-edge innovations improving efficiency, accuracy, and accessibility. One of the most impactful advancements is the integration of digital biomarkers and wearable technologies, which allow real-time monitoring of neurological function, providing objective and continuous data collection. AI and machine learning are also playing a pivotal role in neurology trials, streamlining patient recruitment, predicting treatment responses, and optimizing trial endpoints. Virtual and decentralized clinical trials (DCTs) are gaining momentum, reducing the need for in-person visits and expanding patient participation beyond traditional geographic limitations. Advanced neuroimaging techniques such as functional MRI (fMRI), diffusion tensor imaging (DTI), and PET scans are enhancing the ability to track disease progression and treatment effects with greater precision. Additionally, the development of patient-specific organoid models and in-vitro neurological disease models is accelerating preclinical drug discovery, reducing reliance on traditional animal models. The rise of blockchain technology in clinical trial data management is also improving transparency, data integrity, and patient trust in neurology research. As these technological advancements continue to evolve, neurology clinical trials are becoming more efficient, scalable, and capable of delivering breakthrough therapies for complex neurological conditions.What Is Driving the Growth of the Neurology Clinical Trials Market?
The growth in the neurology clinical trials market is driven by several factors, including the rising prevalence of neurological disorders, increasing investments in neuroscience research, and advancements in precision medicine. The global increase in Alzheimer’ s disease, Parkinson’ s disease, epilepsy, and rare neurological conditions has created an urgent need for effective treatments, prompting pharmaceutical companies and research institutions to accelerate clinical trial efforts. Government initiatives and funding programs aimed at supporting neuroscience research are also fueling market expansion, with regulatory agencies providing incentives for the development of novel CNS-targeted therapies. The shift toward biomarker-driven clinical trials is another key growth driver, enabling better patient stratification and improving trial success rates. The expansion of decentralized clinical trials and virtual trial platforms is further enhancing accessibility, allowing more diverse patient populations to participate in neurology studies. Additionally, collaborations between biotech firms, academic institutions, and contract research organizations (CROs) are driving innovation in trial design and execution. As artificial intelligence, neuroimaging, and digital health technologies continue to reshape the clinical trials landscape, the neurology sector is poised for significant advancements, offering new hope for patients suffering from debilitating neurological disorders.Report Scope
The report analyzes the Neurology Clinical Trials market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Phase Type (Phase I, Phase II, Phase III, Phase IV); Study Design Type (Interventional Study Design, Observational Study Design, Expanded Access Study Design); Indication Type (Epilepsy, Parkinson's Disease, Huntington's Disease, Stroke, Traumatic Brain Injury, Amyotrophic Lateral Sclerosis, Muscle Regeneration, Other Indication Types).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Phase I Clinical Trials segment, which is expected to reach US$3.0 Billion by 2030 with a CAGR of a 5.4%. The Phase II Clinical Trials segment is also set to grow at 3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.5 Billion in 2024, and China, forecasted to grow at an impressive 8.1% CAGR to reach $1.5 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Neurology Clinical Trials Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Neurology Clinical Trials Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Neurology Clinical Trials Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abcam plc, Agilent Technologies, Inc., Beckman Coulter Diagnostics, BioNTech SE, Bio-Rad Laboratories, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Neurology Clinical Trials market report include:
- AbbVie Inc.
- Athira Pharma, Inc.
- Aurora Health Care
- Biogen Inc.
- Charles River Laboratories International, Inc.
- Clarity Pharmaceuticals Ltd
- EBR Systems, Inc.
- Eisai Co., Ltd.
- GlaxoSmithKline plc
- ICON plc
- IQVIA Holdings Inc.
- Medpace Holdings, Inc.
- Merck & Co., Inc.
- Neuren Pharmaceuticals Limited
- Novartis International AG
- PPD (Thermo Fisher Scientific Inc.)
- PYC Therapeutics Limited
- Sanofi S.A.
- Syneos Health
- Telix Pharmaceuticals Limited
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Athira Pharma, Inc.
- Aurora Health Care
- Biogen Inc.
- Charles River Laboratories International, Inc.
- Clarity Pharmaceuticals Ltd
- EBR Systems, Inc.
- Eisai Co., Ltd.
- GlaxoSmithKline plc
- ICON plc
- IQVIA Holdings Inc.
- Medpace Holdings, Inc.
- Merck & Co., Inc.
- Neuren Pharmaceuticals Limited
- Novartis International AG
- PPD (Thermo Fisher Scientific Inc.)
- PYC Therapeutics Limited
- Sanofi S.A.
- Syneos Health
- Telix Pharmaceuticals Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 385 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
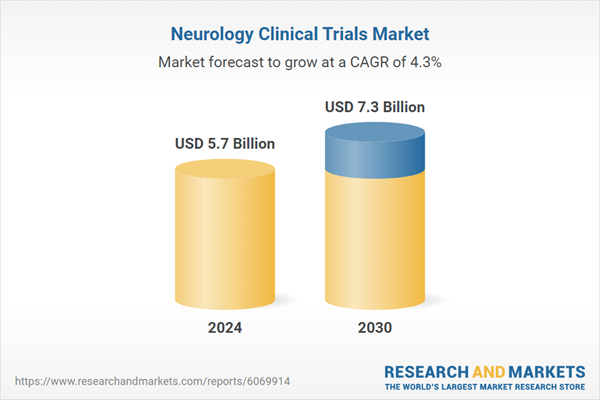
| Estimated Market Value ( USD | $ 5.7 Billion |
| Forecasted Market Value ( USD | $ 7.3 Billion |
| Compound Annual Growth Rate | 4.3% |
| Regions Covered | Global |