Global Intravenous Infusion Pump Market - Key Trends & Drivers Summarized
How Are Intravenous Infusion Pumps Transforming Patient Care?
The intravenous (IV) infusion pump market is experiencing significant growth due to the increasing demand for precision drug delivery, automation in healthcare, and advancements in infusion technology. IV infusion pumps are critical medical devices used to deliver fluids, medications, and nutrients directly into a patient’ s bloodstream in controlled amounts. They play a vital role in hospitals, ambulatory surgical centers, home healthcare, and intensive care units (ICUs) for conditions requiring continuous drug infusion, chemotherapy, pain management, and hydration therapy.With the rising prevalence of chronic diseases such as diabetes, cancer, and cardiovascular disorders, IV infusion pumps have become essential for long-term patient management. Additionally, technological innovations such as smart infusion pumps, wireless connectivity, and AI-driven dosage adjustments are revolutionizing hospital workflows, patient safety, and drug administration accuracy.
The COVID-19 pandemic further accelerated the adoption of IV infusion pumps, as hospitals faced an increased demand for automated and remotely monitored infusion systems to minimize nurse workload, enhance infection control, and optimize patient management in ICU settings. As healthcare providers continue to prioritize automation, patient safety, and integration with digital health systems, the IV infusion pump market is expected to grow rapidly in the coming years.
What Are the Key Trends Driving the IV Infusion Pump Market?
One of the most transformative trends in the IV infusion pump market is the rise of smart infusion pumps. These AI-driven and programmable infusion systems come with dose error reduction systems (DERS), real-time drug libraries, and wireless connectivity, ensuring precise medication administration while reducing human errors. Smart pumps are increasingly being integrated with electronic health records (EHRs) and clinical decision support systems (CDSS) to improve workflow automation and optimize medication dosing.Another major trend is the increasing adoption of ambulatory infusion pumps for home-based therapy. As home healthcare and outpatient treatment gain popularity, lightweight, portable, and wearable infusion pumps are being used for chronic disease management, chemotherapy, and pain relief treatments. This trend aligns with the shift toward personalized and patient-centric care models, where individuals receive treatment outside of traditional hospital settings.
The integration of wireless monitoring and IoT-enabled infusion pumps is also revolutionizing the industry. Modern IV infusion pumps transmit real-time data to hospital networks and cloud-based platforms, allowing remote monitoring of infusion rates, early detection of dosage discrepancies, and predictive maintenance alerts. This technology enhances patient safety, reduces nurse workload, and minimizes medication errors.
Additionally, robotic-assisted infusion pumps and AI-powered dosing algorithms are being developed to improve drug administration accuracy and prevent overdose risks. AI-driven infusion systems analyze patient vitals, drug interactions, and historical dosage patterns to adjust infusion rates dynamically, particularly in critical care and anesthesia management.
Another emerging trend is the development of disposable and needle-free infusion systems. Concerns over infection control, cross-contamination, and needle-stick injuries have driven manufacturers to introduce single-use and closed-system infusion pumps, particularly for oncology, immunotherapy, and neonatal care applications. These innovations align with hospital safety protocols and infection prevention guidelines.
What Challenges Are Impacting the IV Infusion Pump Market?
Despite the rapid advancements in IV infusion technology, the market faces several challenges, including high device costs, maintenance complexity, and regulatory hurdles. One of the biggest concerns is the high cost of smart infusion pumps and advanced drug delivery systems. While these devices enhance accuracy and safety, they require significant investment in hospital infrastructure, training, and cybersecurity, making adoption difficult for small and mid-sized healthcare facilities.Another key challenge is infusion pump errors and device malfunctions. Programming errors, mechanical failures, and software glitches have resulted in serious medication errors, overdoses, and under-dosing incidents, leading to product recalls and regulatory scrutiny. The FDA and other health agencies have issued safety alerts regarding infusion pump malfunctions, prompting manufacturers to improve quality control, error prevention algorithms, and user training.
Cybersecurity threats and data vulnerabilities pose another challenge, as IoT-enabled and wireless infusion pumps become more common. Hackers targeting hospital networks could manipulate infusion rates or compromise patient data, leading to serious health risks and liability concerns. Healthcare facilities must implement robust cybersecurity protocols, encryption technologies, and regular software updates to prevent potential cyberattacks on infusion devices.
Complexity in device interoperability and integration with hospital IT systems also hinders adoption. Many hospitals still operate with legacy electronic health record (EHR) systems, making it difficult to seamlessly integrate smart IV pumps with existing infrastructure. Standardization efforts are needed to ensure smooth data exchange and compatibility across different healthcare platforms.
Additionally, stringent regulatory approvals and compliance requirements delay the introduction of new infusion pump models. IV infusion pumps must meet strict FDA, CE (Europe), and ISO (International Organization for Standardization) guidelines regarding safety, dosage accuracy, and software reliability. The lengthy approval process increases R&D costs and time-to-market for innovative infusion devices.
What Factors Are Driving the Growth of the IV Infusion Pump Market?
The growth in the IV infusion pump market is driven by several factors, including the rising prevalence of chronic diseases, advancements in medical technology, and increasing demand for automation in drug delivery. One of the biggest drivers is the growing need for precise and controlled medication administration, particularly in critical care, oncology, and neonatal care. Smart infusion pumps equipped with safety mechanisms help reduce medication errors, making them essential in high-risk environments such as ICUs, operating rooms, and pediatric wards.Another major driver is the increasing adoption of home-based and ambulatory infusion therapy. Patients with chronic conditions such as diabetes, cancer, and autoimmune disorders often require long-term infusion therapy, leading to the rising demand for portable and wearable IV pumps that enable treatment outside hospital settings. The growing elderly population and the rise of telehealth services further support this trend.
The increasing demand for parenteral nutrition and pain management solutions is also propelling market growth. Patients suffering from malnutrition, gastrointestinal disorders, or post-operative recovery require intravenous feeding via infusion pumps, driving demand for specialized enteral and parenteral infusion devices. Similarly, patient-controlled analgesia (PCA) pumps are gaining popularity for opioid-free pain management in post-surgical and palliative care.
Government initiatives and healthcare infrastructure modernization projects in emerging markets are also fueling market expansion. Countries across Asia-Pacific, Latin America, and the Middle East are investing in advanced hospital equipment, digital health solutions, and smart infusion systems to enhance patient care and reduce hospital-acquired complications.
Furthermore, the rise of personalized medicine and biologic drug delivery is driving demand for specialized infusion systems designed for gene therapy, immunotherapy, and targeted cancer treatments. With the growing pipeline of complex biologic drugs, pharmaceutical companies are partnering with medical device manufacturers to develop next-generation infusion technologies for precision drug administration.
Conclusion: The Future of IV Infusion Pump Technology
The IV infusion pump market is poised for continued growth as healthcare facilities, home-based care providers, and pharmaceutical companies adopt smarter, safer, and more efficient drug delivery systems. With ongoing innovations in AI, robotics, and digital health integration, IV pumps will play an increasingly critical role in personalized medicine, automated drug administration, and remote patient monitoring.Manufacturers that prioritize device safety, cybersecurity, regulatory compliance, and seamless hospital integration will gain a competitive edge in the evolving infusion therapy landscape. As demand for precision-based, minimally invasive, and connected infusion systems rises, the future of IV therapy will be defined by automation, patient-centric solutions, and advanced digital health ecosystems.
Report Scope
The report analyzes the Intravenous Infusion Pumps market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Volumetric Infusion Pumps, Insulin Infusion Pumps, Syringe Infusion Pumps, Enteral Infusion Pumps, Ambulatory Infusion Pumps, Patient Controlled Analgesia, Implantable Infusion Pumps, Others); Disease Indication (Chemotherapy, Diabetes, Gastroenterology, Analgesia / Pain Management, Pediatrics / Neonatology, Hematology, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Volumetric Infusion Pumps segment, which is expected to reach US$2.5 Billion by 2030 with a CAGR of a 5.1%. The Insulin Infusion Pumps segment is also set to grow at 5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.6 Billion in 2024, and China, forecasted to grow at an impressive 9% CAGR to reach $1.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Intravenous Infusion Pumps Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Intravenous Infusion Pumps Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Intravenous Infusion Pumps Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Baxter International, Fresenius Kabi, B. Braun Melsungen AG, ICU Medical, Grifols and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Intravenous Infusion Pumps market report include:
- Alaris (part of BD)
- Autoinfu
- B. Braun Infusomat
- B. Braun Melsungen AG
- Baxter International
- Baxter Sigma Spectrum
- BD (Becton, Dickinson and Company)
- Biomedix Medical, Inc.
- DiaMedical USA
- Fresenius Kabi
- ICU Medical
- IRadimed Corporation
- Med One Group
- Medtronic plc
- Moog Inc.
- Moog Medical Devices Group
- Smiths Medical
- Terumo Corporation
- Zyno Medical
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alaris (part of BD)
- Autoinfu
- B. Braun Infusomat
- B. Braun Melsungen AG
- Baxter International
- Baxter Sigma Spectrum
- BD (Becton, Dickinson and Company)
- Biomedix Medical, Inc.
- DiaMedical USA
- Fresenius Kabi
- ICU Medical
- IRadimed Corporation
- Med One Group
- Medtronic plc
- Moog Inc.
- Moog Medical Devices Group
- Smiths Medical
- Terumo Corporation
- Zyno Medical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 307 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
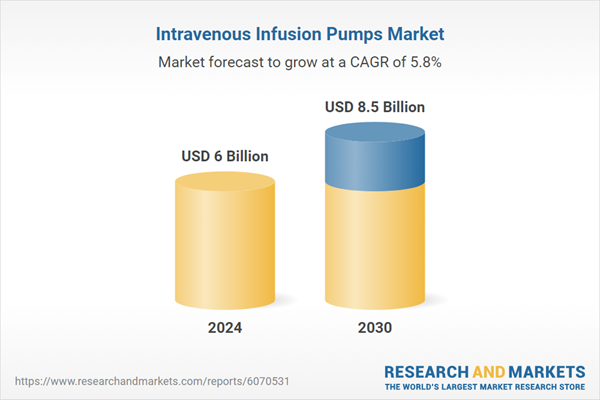
| Estimated Market Value ( USD | $ 6 Billion |
| Forecasted Market Value ( USD | $ 8.5 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |