Global Medical Grade Ultra High Molecular Weight Polyethylene Monomers Market - Key Trends & Drivers Summarized
Is This Super Polymer Reshaping the Future of Implantable Medical Devices?
Medical grade ultra high molecular weight polyethylene (UHMWPE) monomers are rapidly becoming foundational in the design and manufacturing of high-performance implantable medical devices. Unlike conventional polymer materials, UHMWPE stands out due to its exceptional biocompatibility, superior impact resistance, low friction coefficient, and remarkable wear performance - attributes that are indispensable in orthopedics, trauma fixation, and cardiovascular applications. Historically, UHMWPE gained prominence as the bearing surface material in total joint arthroplasty, particularly in hip and knee replacements. Today, its utility extends into spinal implants, acetabular liners, tibial inserts, cranial stabilization plates, and more recently, in articulating components for robotic-assisted surgeries. The ultra-long chains of polyethylene molecules in UHMWPE provide mechanical strength and oxidative stability, reducing implant loosening and the generation of wear particles over time. These characteristics are especially critical in orthopedic implants, where joint longevity is a top priority, particularly for younger and more active patients. The growing demand for minimally invasive and customized implants is driving interest in modified UHMWPE grades enhanced with vitamin E for oxidation resistance, cross-linking for wear reduction, or doped with antimicrobial agents to mitigate infection risks. The medical-grade monomers, which serve as the raw building blocks for these specialized polymer forms, are witnessing escalating demand as material innovation becomes a key differentiator in next-generation implantable devices. As surgeons and hospitals increasingly demand longer-lasting, safer, and performance-optimized biomaterials, UHMWPE monomers are transitioning from a niche product into a mainstream requirement in advanced medical device manufacturing.What Are the Key Technological Advancements That Are Unlocking New Applications?
Innovation in polymer science and processing technologies is significantly expanding the scope of medical grade UHMWPE monomers across diverse healthcare applications. One of the most critical advancements has been in the field of highly cross-linked UHMWPE, which exhibits drastically improved wear resistance, making it especially suitable for high-load-bearing implants like knee and hip prostheses. Modern cross-linking techniques, including gamma irradiation followed by thermal annealing, ensure uniform molecular architecture without compromising the material's tensile strength or fatigue resistance. Additionally, the introduction of antioxidant-stabilized UHMWPE - particularly vitamin E-infused formulations - has mitigated long-standing concerns regarding in vivo oxidative degradation. These improvements have led to extended implant lifespan, a reduction in revision surgeries, and better long-term clinical outcomes. Advances in machining and precision fabrication have also enabled the use of UHMWPE in patient-specific orthopedic solutions, allowing customized liners and spacers that conform to individual anatomy and joint kinematics. Another breakthrough is the growing use of UHMWPE in the development of surgical sutures, vascular patches, and maxillofacial implants, where the combination of mechanical durability and biological inertness offers a superior alternative to conventional materials. Furthermore, additive manufacturing (3D printing) is slowly gaining traction, with experimental successes in forming UHMWPE-based scaffolds and porous structures for osseointegration. As regulatory agencies continue to evaluate long-term safety profiles, these innovations are laying the groundwork for broader adoption in both load-bearing and non-load-bearing applications. Technology has transformed UHMWPE from a singular orthopedic solution to a versatile biomaterial with cross-disciplinary relevance across surgical specialties.Why Are End-Use Segments Prioritizing UHMWPE Monomers Over Conventional Polymers?
The growing reliance on UHMWPE monomers in end-use medical segments is largely driven by the evolving performance expectations of both clinicians and patients. Orthopedic device manufacturers, in particular, are emphasizing longevity and biointegration, as healthcare providers shift focus toward value-based care models that reward outcomes over procedural volumes. The predictable wear behavior and biological inertness of UHMWPE make it ideal for critical applications where mechanical stress and body fluid exposure are constant. Beyond orthopedics, neurosurgery and cardiovascular care are rapidly adopting UHMWPE components due to their low particulate generation and compatibility with minimally invasive delivery systems. The home healthcare and ambulatory surgical sectors are also creating new use cases, with demand rising for lightweight, durable, and biocompatible components in devices like joint braces, prosthetics, and portable surgical kits. The dental implant market is another emerging frontier where UHMWPE monomers are being explored for temporary abutments and baseplates owing to their resilience and non-reactive properties. Additionally, hospitals and surgical centers are under pressure to standardize device procurement while ensuring performance consistency and patient safety - pushing OEMs to opt for materials like UHMWPE that are backed by years of clinical data and regulatory approvals. Custom implant production is further influencing material selection, with UHMWPE being favored for CNC machining compatibility and batch consistency. As device manufacturers contend with increasing regulatory scrutiny, especially for Class III implantables, the traceability and certification pedigree of medical-grade UHMWPE monomers become non-negotiable. This convergence of clinical demand, economic viability, and regulatory alignment is making UHMWPE the material of choice across a widening spectrum of medical device applications.The Growth in the Medical Grade Ultra High Molecular Weight Polyethylene Monomers Market Is Driven by Several Factors…
The growth in the medical grade ultra high molecular weight polyethylene monomers market is driven by several factors rooted in material innovation, expanding end-use integration, and increasing patient preference for advanced medical implants. A major driver is the widespread shift toward highly cross-linked and antioxidant-stabilized polyethylene formulations, which have significantly extended implant life and reduced revision rates in joint replacement procedures. Technological advancements in polymer engineering and machining are enabling the creation of thinner, more anatomically precise implant components, which is fueling demand for high-purity UHMWPE monomers used in both standard and patient-specific devices. The rising volume of orthopedic surgeries - driven by aging populations, sports injuries, and lifestyle-related joint disorders - is directly expanding the consumption of UHMWPE-based implantable materials. Additionally, the proliferation of robotic-assisted surgical systems has created a need for more dimensionally stable and wear-resistant biomaterials that can operate seamlessly within highly precise, repeatable mechanical environments. In parallel, the rapid expansion of minimally invasive and outpatient procedures is accelerating the adoption of lightweight, biocompatible polymers, especially in emerging markets where access to follow-up care is limited. Consumer behavior trends also play a role, with patients increasingly opting for advanced implant technologies that promise longer performance life and minimal post-surgical complications. On the regulatory front, consistent classification and traceability standards for medical-grade UHMWPE monomers - especially in North America and the EU - are encouraging more OEMs and Tier 1 suppliers to integrate this material across product lines. Furthermore, the rising popularity of hybrid implant designs, combining metals with polymeric interfaces, is creating hybrid demand streams for UHMWPE as a critical interface component. The growth trajectory is also supported by ongoing collaborations between polymer manufacturers and device makers to co-develop monomer blends optimized for specific clinical applications. These developments, together with the strategic prioritization of patient safety, surgical efficiency, and long-term outcomes, are driving robust and sustained growth in the global medical-grade UHMWPE monomers market.Report Scope
The report analyzes the Medical Grade Ultra High Molecular Weight Polyethylene Monomers market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Application (hip replacement, Knee replacement, Shoulder replacement, Ankle replacement, Small joints).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Hip Replacement Application segment, which is expected to reach US$2.9 Billion by 2030 with a CAGR of a 9.3%. The Knee Replacement Application segment is also set to grow at 9.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.3 Billion in 2024, and China, forecasted to grow at an impressive 13.4% CAGR to reach $1.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Medical Grade Ultra High Molecular Weight Polyethylene Monomers Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Medical Grade Ultra High Molecular Weight Polyethylene Monomers Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Medical Grade Ultra High Molecular Weight Polyethylene Monomers Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Asahi Kasei Corporation, Celanese Corporation, CPS GmbH, Crown Plastics Co., Inc., Curbell Plastics, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Medical Grade Ultra High Molecular Weight Polyethylene Monomers market report include:
- Asahi Kasei Corporation
- Celanese Corporation
- CPS GmbH
- Crown Plastics Co., Inc.
- Curbell Plastics, Inc.
- Dotmar Engineering Plastics
- DSM Biomedical
- Ensinger GmbH
- Garland Manufacturing Company
- Honeywell International Inc.
- LyondellBasell Industries Holdings B.V.
- Mitsui Chemicals, Inc.
- Orthoplastics Ltd.
- Quadrant Engineering Plastic Products (Mitsubishi Chemical Advanced Materials)
- Rochling Group
- Shandong Ningjin Xinxing Chemical Co., Ltd.
- Sunwell Global Ltd.
- Teijin Limited
- Tianyi Biomedical Tech Pte. Ltd.
- UltraPoly Corporation
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Asahi Kasei Corporation
- Celanese Corporation
- CPS GmbH
- Crown Plastics Co., Inc.
- Curbell Plastics, Inc.
- Dotmar Engineering Plastics
- DSM Biomedical
- Ensinger GmbH
- Garland Manufacturing Company
- Honeywell International Inc.
- LyondellBasell Industries Holdings B.V.
- Mitsui Chemicals, Inc.
- Orthoplastics Ltd.
- Quadrant Engineering Plastic Products (Mitsubishi Chemical Advanced Materials)
- Rochling Group
- Shandong Ningjin Xinxing Chemical Co., Ltd.
- Sunwell Global Ltd.
- Teijin Limited
- Tianyi Biomedical Tech Pte. Ltd.
- UltraPoly Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
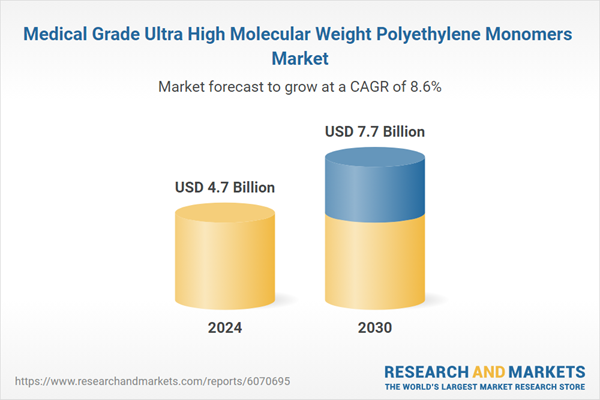
| Estimated Market Value ( USD | $ 4.7 Billion |
| Forecasted Market Value ( USD | $ 7.7 Billion |
| Compound Annual Growth Rate | 8.6% |
| Regions Covered | Global |