Global Dendritic Cell Cancer Vaccines Market - Key Trends & Drivers Summarized
Why Are Dendritic Cell Cancer Vaccines Gaining Attention in Immunotherapy?
Dendritic cell (DC) cancer vaccines have emerged as a promising frontier in oncology, leveraging the body’ s immune system to combat malignancies. Unlike traditional cancer treatments such as chemotherapy and radiation, which often have severe side effects, DC vaccines offer a personalized and targeted approach to cancer therapy. These vaccines work by priming dendritic cells with tumor antigens, enabling them to activate T-cells for an enhanced immune response against cancer cells. The growing recognition of immunotherapy as a viable cancer treatment has propelled research and investment in dendritic cell-based vaccines. The increasing incidence of cancers such as melanoma, prostate, lung, and glioblastoma has further fueled interest in personalized vaccine strategies. Additionally, breakthroughs in cancer genomics and molecular biology are improving vaccine efficacy, offering new hope for patients with aggressive and treatment-resistant cancers. As research progresses, DC vaccines are being evaluated for their potential to complement existing immunotherapies, such as checkpoint inhibitors and CAR-T cell therapy, creating a more comprehensive and durable cancer treatment landscape.How Are Advancements in Biotechnology Enhancing DC Vaccine Development?
The development of dendritic cell cancer vaccines has been significantly enhanced by advancements in biotechnology, including gene editing, synthetic biology, and nanotechnology. CRISPR-based gene editing has enabled researchers to engineer dendritic cells with enhanced antigen presentation capabilities, improving their effectiveness in stimulating the immune system. Additionally, the use of messenger RNA (mRNA) technology - popularized by COVID-19 vaccines - is being explored for developing next-generation DC vaccines with more efficient tumor antigen loading. Innovations in nanocarriers and lipid nanoparticles have also improved vaccine delivery, enhancing the bioavailability and stability of therapeutic dendritic cells. Furthermore, artificial intelligence (AI) and big data analytics are being employed to identify novel cancer antigens, optimizing the personalization of DC vaccines for different tumor types. As these technological advancements continue to evolve, they are expected to drive the development of more effective and scalable dendritic cell cancer vaccine platforms.What Are the Regulatory and Market Challenges Facing DC Vaccines?
Despite their potential, dendritic cell cancer vaccines face several regulatory and commercial challenges. The high cost and complexity of personalized vaccine manufacturing remain significant hurdles, as each vaccine must be tailored to an individual patient’ s tumor profile. Additionally, the stringent regulatory approval process for cellular immunotherapies requires extensive clinical trials and long-term efficacy data, delaying market entry. The lack of standardized protocols for dendritic cell preparation and antigen loading has also led to variability in vaccine effectiveness across different patient groups. However, regulatory agencies such as the FDA and EMA are increasingly supporting immunotherapy research, providing fast-track designations for promising DC vaccine candidates. Additionally, biotech companies and research institutions are forming strategic partnerships to scale up production and reduce costs. With continued investment in automation and bioprocessing technologies, the challenges surrounding DC vaccine commercialization are expected to be gradually mitigated.What Are the Key Growth Drivers in the Dendritic Cell Cancer Vaccines Market?
The growth in the dendritic cell cancer vaccines market is driven by several factors, including the rising global cancer burden and increasing demand for personalized immunotherapy solutions. Advances in biotechnology, particularly in gene editing, mRNA technology, and nanomedicine, have significantly enhanced the efficacy and scalability of DC vaccines. The growing adoption of combination immunotherapies, integrating DC vaccines with checkpoint inhibitors and monoclonal antibodies, has further expanded treatment possibilities. Regulatory support for cellular immunotherapies, including expedited approval pathways, has accelerated clinical trials and market entry for new DC vaccine candidates. Additionally, increased funding from government agencies, research institutions, and biotech firms has driven innovation and commercialization efforts. As the field of cancer immunotherapy continues to evolve, dendritic cell cancer vaccines are expected to play a critical role in the future of oncology, offering a targeted and durable solution for patients with hard-to-treat cancers.Report Scope
The report analyzes the Dendritic Cell Cancer Vaccines market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Sipuleucel-T Vaccines, CreaVax Vaccines, Other Products); End-Use (Adults Vaccines End-Use, Pediatrics Vaccines End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Sipuleucel-T Vaccines segment, which is expected to reach US$987.7 Million by 2030 with a CAGR of a 14.7%. The CreaVax Vaccines segment is also set to grow at 12% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $211.9 Million in 2024, and China, forecasted to grow at an impressive 12.1% CAGR to reach $259.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Dendritic Cell Cancer Vaccines Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Dendritic Cell Cancer Vaccines Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Dendritic Cell Cancer Vaccines Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alcon Inc., Allergan (AbbVie Inc.), Aurolab, Bausch Health Companies Inc., Entod Pharmaceuticals Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Dendritic Cell Cancer Vaccines market report include:
- Argos Therapeutics, Inc.
- Asgard Therapeutics
- BioNTech SE
- Candel Therapeutics
- CureVac N.V.
- Dendreon Pharmaceuticals LLC
- Diakonos Oncology
- Genexine, Inc.
- GlaxoSmithKline plc (GSK)
- ImmunoCellular Therapeutics, Ltd.
- Immunomic Therapeutics, Inc.
- Medigene AG
- Mestag Therapeutics
- Mill Creek Life Sciences
- Moderna, Inc.
- Northwest Biotherapeutics
- PDC*line Pharma
- Scancell Holdings plc
- SOTIO Biotech
- TransImmune AG
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Argos Therapeutics, Inc.
- Asgard Therapeutics
- BioNTech SE
- Candel Therapeutics
- CureVac N.V.
- Dendreon Pharmaceuticals LLC
- Diakonos Oncology
- Genexine, Inc.
- GlaxoSmithKline plc (GSK)
- ImmunoCellular Therapeutics, Ltd.
- Immunomic Therapeutics, Inc.
- Medigene AG
- Mestag Therapeutics
- Mill Creek Life Sciences
- Moderna, Inc.
- Northwest Biotherapeutics
- PDC*line Pharma
- Scancell Holdings plc
- SOTIO Biotech
- TransImmune AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 142 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
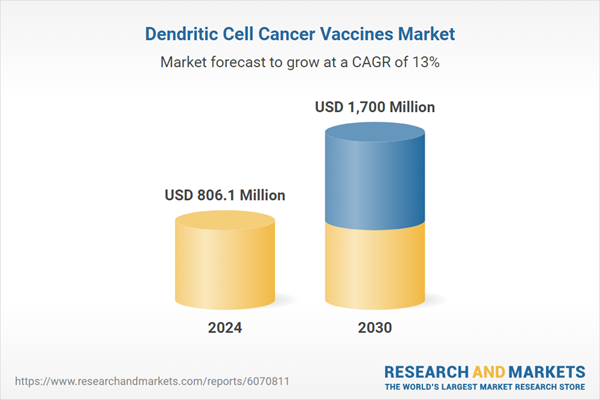
| Estimated Market Value ( USD | $ 806.1 Million |
| Forecasted Market Value ( USD | $ 1700 Million |
| Compound Annual Growth Rate | 13.0% |
| Regions Covered | Global |