Global Intravascular Catheters Market - Key Trends & Drivers Summarized
How Are Intravascular Catheters Revolutionizing Medical Procedures?
The intravascular catheters market is witnessing significant growth due to increasing demand for minimally invasive procedures, advancements in catheter technology, and rising prevalence of chronic diseases requiring vascular access. Intravascular catheters are essential for a wide range of medical applications, including drug administration, fluid infusion, hemodialysis, cardiovascular interventions, and critical care monitoring. These catheters provide safe, efficient, and precise access to blood vessels, reducing complications associated with traditional surgical interventions.With the global rise in cardiovascular diseases (CVDs), cancer treatments, and end-stage renal disease (ESRD) requiring dialysis, intravascular catheter adoption is increasing across hospitals, ambulatory surgical centers, and home healthcare settings. Additionally, the growth of interventional cardiology, neurology, and radiology has expanded the use of specialized guiding catheters, balloon catheters, and microcatheters for precision-based procedures such as angioplasty, stent placement, and thrombectomy.
Technological advancements, such as antimicrobial-coated catheters, bioabsorbable catheters, and catheter navigation systems, are enhancing infection control, patient safety, and procedural efficiency. The shift toward minimally invasive treatments, coupled with the increasing use of catheter-based therapies in outpatient settings, is expected to drive continued market expansion.
What Are the Key Trends Shaping the Intravascular Catheters Market?
One of the most notable trends in the intravascular catheters market is the development of antimicrobial and heparin-coated catheters. Catheter-associated bloodstream infections (CLABSIs) and thrombosis are major complications in long-term catheter use, leading to increased hospitalizations and mortality rates. To address this, manufacturers are introducing catheters embedded with silver-ion, chlorhexidine, and antimicrobial coatings to reduce infection risks and improve catheter longevity.Another key trend is the integration of smart sensors and real-time monitoring capabilities in catheters. Smart catheters equipped with pressure sensors, wireless connectivity, and microfluidic technology are enabling continuous hemodynamic monitoring, early detection of blockages, and improved fluid management. These innovations are particularly valuable in intensive care units (ICUs) and interventional cardiology, where real-time patient data can significantly improve outcomes.
The increasing adoption of peripherally inserted central catheters (PICCs) and midline catheters is also reshaping the market. PICCs offer an alternative to central venous catheters (CVCs) for long-term intravenous therapy, reducing the need for repeated venipunctures. Midline catheters, which provide intermediate vascular access, are gaining traction in outpatient settings and home healthcare, enabling cost-effective, extended IV therapy without the risks associated with central line infections.
Another emerging trend is the use of biodegradable and bioresorbable catheters. Traditional polymer-based catheters can cause foreign body reactions and thrombosis, whereas biodegradable catheters dissolve over time, minimizing long-term complications. This innovation is particularly beneficial in temporary catheter placements, neurovascular interventions, and pediatric applications where minimizing residual foreign material is critical.
Additionally, the rise in interventional and diagnostic catheterization procedures is driving demand for microcatheters and steerable guiding catheters. These specialized devices are widely used in angioplasty, embolization, neurointerventions, and electrophysiology procedures, allowing precise navigation through complex vascular structures. The increasing adoption of robot-assisted catheter navigation systems is further enhancing accuracy, efficiency, and procedural success rates in catheter-based interventions.
What Challenges Are Impacting the Intravascular Catheters Market?
Despite the numerous advancements in intravascular catheter technology, several challenges hinder widespread adoption and market growth. One of the primary concerns is catheter-related infections and thrombosis, which can lead to serious complications, extended hospital stays, and increased healthcare costs. While antimicrobial coatings and advanced catheter materials help reduce infection risks, strict aseptic techniques and regular catheter maintenance are essential to prevent complications.Another major challenge is the high cost of advanced catheterization devices and procedures. Drug-eluting catheters, bioresorbable catheters, and sensor-integrated catheters come at a premium cost, making them less accessible in low-income regions. Additionally, reimbursement limitations for advanced catheter-based procedures in certain countries can restrict patient access to cutting-edge treatments.
Regulatory hurdles and stringent approval processes pose another challenge for market players. Medical device regulatory authorities such as the FDA (U.S. Food and Drug Administration), EMA (European Medicines Agency), and PMDA (Pharmaceuticals and Medical Devices Agency, Japan) impose strict clinical trial requirements for new catheter technologies, coatings, and materials. The time-consuming approval process can delay product launches and increase development costs.
Material biocompatibility and catheter durability are also key concerns. Catheters made from silicone, polyurethane, and PTFE (polytetrafluoroethylene) must exhibit optimal flexibility, kink resistance, and hemodynamic stability to prevent clot formation and vessel trauma. Balancing softness with durability while ensuring low thrombogenicity remains a technical challenge for manufacturers.
Additionally, the shortage of skilled healthcare professionals trained in advanced catheterization procedures is a limiting factor in some regions. Interventional cardiologists, radiologists, and vascular surgeons require specialized training to handle complex catheter-based interventions, and the lack of expertise can impact procedural success rates.
What Factors Are Driving the Growth of the Intravascular Catheters Market?
The growth in the intravascular catheters market is driven by several factors, including the rising incidence of cardiovascular diseases, the increasing use of minimally invasive interventions, and continuous innovations in catheter technology. One of the major drivers is the growing prevalence of cardiovascular disorders, such as coronary artery disease (CAD), stroke, and peripheral artery disease (PAD), which require catheter-based interventions such as angioplasty, stenting, and thrombectomy.Another key driver is the expanding applications of catheters in oncology, nephrology, and neurology. Catheter-based chemotherapy infusion, dialysis catheters for end-stage renal disease (ESRD), and neurovascular catheters for stroke treatment are contributing to market growth. The rising global burden of cancer and kidney diseases is driving demand for long-term vascular access devices.
The increasing adoption of outpatient and home-based infusion therapies is further fueling market expansion. Peripherally inserted central catheters (PICCs) and midline catheters are being widely used for long-term antibiotic therapy, chemotherapy, and parenteral nutrition, allowing patients to receive treatment outside of hospital settings. This trend is particularly significant in chronic disease management and elderly patient care, where home-based IV therapy reduces hospital admissions and healthcare costs.
Technological advancements, such as robotic-assisted catheter navigation, real-time pressure-sensing catheters, and 3D imaging-assisted catheter placement, are enhancing the precision and safety of minimally invasive vascular procedures. The introduction of AI-driven catheter placement guidance systems is helping reduce complications, improve procedural outcomes, and shorten procedure times.
Additionally, government initiatives and healthcare investments in improving vascular access care and catheter-based treatment infrastructure are supporting market growth. Many developing countries are expanding interventional cardiology and dialysis centers, increasing the adoption of high-quality intravascular catheters and vascular access solutions.
With ongoing advancements in catheter coatings, biodegradable materials, smart sensors, and AI-driven imaging, the intravascular catheters market is poised for continuous growth and innovation. Companies that focus on infection control, cost-effective solutions, and regulatory compliance will gain a competitive advantage in meeting the evolving needs of healthcare providers and patients worldwide.
Report Scope
The report analyzes the Intravascular Catheters market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Short PIVC, Integrated / Closed PIVC); Application (Oncology, Gastroenterology, Renal Disease, Infectious Diseases, Others); End-Use (Hospitals, Clinics, Ambulatory Surgery Centers, Homecare, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Short PIVC segment, which is expected to reach US$10.9 Billion by 2030 with a CAGR of a 13.4%. The Integrated / Closed PIVC segment is also set to grow at 8.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.1 Billion in 2024, and China, forecasted to grow at an impressive 16.4% CAGR to reach $3.2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Intravascular Catheters Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Intravascular Catheters Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Intravascular Catheters Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ACTEON, Air Techniques, Biolase, Carestream Dental, Dentsply Sirona and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Intravascular Catheters market report include:
- AMECATH
- AngioDynamics, Inc.
- Angiplast Pvt. Ltd.
- Argon Medical Devices, Inc.
- Asahi Kasei Corporation
- B. Braun Melsungen AG
- Becton, Dickinson and Company (BD)
- Boston Scientific Corporation
- Cook Medical
- Edwards Lifesciences Corporation
- Hindustan Syringes & Medical Devices Ltd.
- ICU Medical, Inc.
- Johnson & Johnson
- McKesson Corporation
- Medline Industries, Inc.
- Medtronic PLC
- Nipro Medical Corporation
- PRODiMED
- Romsons Scientific & Surgical Pvt. Ltd.
- Smiths Medical
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AMECATH
- AngioDynamics, Inc.
- Angiplast Pvt. Ltd.
- Argon Medical Devices, Inc.
- Asahi Kasei Corporation
- B. Braun Melsungen AG
- Becton, Dickinson and Company (BD)
- Boston Scientific Corporation
- Cook Medical
- Edwards Lifesciences Corporation
- Hindustan Syringes & Medical Devices Ltd.
- ICU Medical, Inc.
- Johnson & Johnson
- McKesson Corporation
- Medline Industries, Inc.
- Medtronic PLC
- Nipro Medical Corporation
- PRODiMED
- Romsons Scientific & Surgical Pvt. Ltd.
- Smiths Medical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 384 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
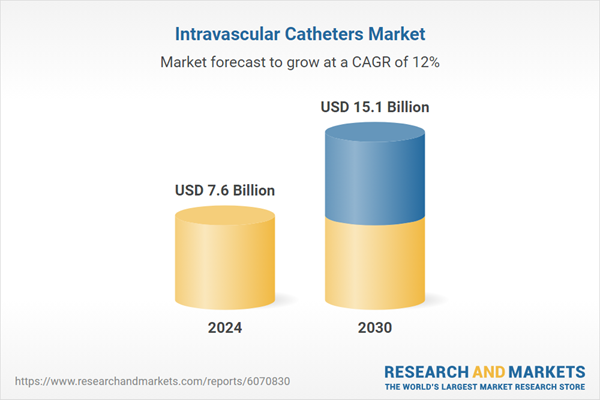
| Estimated Market Value ( USD | $ 7.6 Billion |
| Forecasted Market Value ( USD | $ 15.1 Billion |
| Compound Annual Growth Rate | 12.0% |
| Regions Covered | Global |