Global GI Stool Testing Market - Key Trends & Drivers Summarized
Why Is GI Stool Testing Becoming an Essential Diagnostic Tool?
Gastrointestinal (GI) stool testing has become an indispensable diagnostic method for detecting a wide range of digestive disorders, infections, and inflammatory conditions. This non-invasive testing approach allows healthcare professionals to analyze stool samples for the presence of pathogens, blood, digestive enzymes, and biomarkers associated with gastrointestinal diseases. As gut health plays a crucial role in overall wellness, the demand for accurate and accessible stool testing solutions has grown significantly in recent years.Advancements in microbiome analysis and molecular diagnostic techniques have further enhanced the accuracy and scope of GI stool testing. Tests such as fecal occult blood tests (FOBT), fecal calprotectin assays, and multiplex PCR-based stool pathogen detection have improved early diagnosis of colorectal cancer, inflammatory bowel disease (IBD), and bacterial infections. With the rise of functional medicine and personalized healthcare, stool testing is also being used to assess gut microbiota composition, aiding in targeted treatments for conditions such as irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO).
Which Medical Applications Are Driving Demand for GI Stool Testing?
GI stool testing is widely used in gastroenterology for the diagnosis of colorectal cancer, inflammatory bowel disease, and gastrointestinal infections. Fecal immunochemical tests (FIT) and FOBT have become standard tools for colorectal cancer screening, enabling early detection of occult blood in stool samples. Additionally, stool calprotectin and lactoferrin tests are commonly used to distinguish between inflammatory bowel disease and functional GI disorders, reducing the need for invasive procedures such as colonoscopy.Infectious disease diagnostics is another major area where GI stool testing plays a crucial role. Multiplex PCR panels allow for the rapid detection of bacterial, viral, and parasitic infections, improving treatment outcomes and reducing antibiotic misuse. The growing awareness of gut microbiome health has also led to increased demand for stool-based microbiota profiling, providing insights into dysbiosis-related conditions such as obesity, diabetes, and autoimmune disorders. The integration of stool testing into routine health check-ups and precision medicine initiatives is further driving market expansion.
What Are the Latest Technological Innovations in GI Stool Testing?
Recent technological advancements have significantly improved the efficiency, accuracy, and accessibility of GI stool testing. One of the most transformative developments is the adoption of next-generation sequencing (NGS) for gut microbiome analysis, enabling comprehensive profiling of bacterial, fungal, and viral communities within the digestive tract. Additionally, AI-driven diagnostic platforms are being integrated with stool testing, allowing for automated interpretation of results and personalized treatment recommendations.Another key innovation is the development of at-home stool test kits, which allow patients to collect and send samples for laboratory analysis without visiting healthcare facilities. These direct-to-consumer tests have increased accessibility to gastrointestinal health assessments, particularly for preventive screenings and microbiome testing. The use of CRISPR-based diagnostic methods for detecting specific pathogens in stool samples is also emerging, offering ultra-sensitive and rapid detection capabilities. These advancements are making GI stool testing more precise, user-friendly, and clinically valuable.
What Factors Are Fueling the Growth of the GI Stool Testing Market?
The growth in the GI stool testing market is driven by several factors, including rising prevalence of gastrointestinal diseases, increasing adoption of non-invasive diagnostics, and technological advancements in molecular testing. The global burden of colorectal cancer, inflammatory bowel diseases, and gut microbiome-related disorders has fueled demand for early and accurate diagnostic solutions. Government-led colorectal cancer screening programs have further boosted the adoption of stool-based tests.The expansion of microbiome research and the growing consumer interest in gut health have also contributed to market growth. Direct-to-consumer microbiome analysis services have gained popularity, offering personalized dietary and probiotic recommendations based on stool test results. Additionally, advancements in portable diagnostic devices and digital health platforms have improved accessibility to stool-based testing in both clinical and at-home settings. As gastrointestinal diagnostics continue to evolve, GI stool testing is expected to play a vital role in precision medicine, disease prevention, and gut health optimization.
Report Scope
The report analyzes the GI Stool Testing market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product (GI Stool Testing Consumables, GI Stool Testing Analyzers); Test (Occult Blood Test, Bacteria Test, Fecal Biomarkers Test, Ova & Parasites Test, Other Test Types); Application (Infection Application, Cancer Application, Inflammatory Bowel Disease Application, Gastroesophageal Reflux Disease Application, Other Applications); Distribution Channel (Brick & Mortar Distribution Channel, E-Commerce Distribution Channel); End-Use (Hospitals & Clinics End-Use, Diagnostic Centers End-Use, Homecare Settings End-Use, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the GI Stool Testing Consumables segment, which is expected to reach US$501.1 Million by 2030 with a CAGR of a 4.7%. The GI Stool Testing Analyzers segment is also set to grow at 7.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $171.9 Million in 2024, and China, forecasted to grow at an impressive 9.2% CAGR to reach $179.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global GI Stool Testing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global GI Stool Testing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global GI Stool Testing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as British Geriatrics Society, Centre of Ageing Better, HelpAge India Non Profit Organisation in India, Keele University, National Ageing Research Institute Limited. (NARI) and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this GI Stool Testing market report include:
- Abbott Laboratories
- Beckman Coulter, Inc.
- bioMérieux SA
- Bio-Rad Laboratories, Inc.
- Cardinal Health, Inc.
- Cenogenics Corporation
- CTK Biotech, Inc.
- Danaher Corporation
- DiaSorin S.p.A.
- Epitope Diagnostics, Inc.
- Exact Sciences Corporation
- Genova Diagnostics
- Hologic, Inc.
- Meridian Bioscience, Inc.
- Nanosphere, Inc.
- Quest Diagnostics
- Roche Diagnostics
- ScheBo Biotech AG
- Siemens Healthineers
- Trinity Biotech
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Beckman Coulter, Inc.
- bioMérieux SA
- Bio-Rad Laboratories, Inc.
- Cardinal Health, Inc.
- Cenogenics Corporation
- CTK Biotech, Inc.
- Danaher Corporation
- DiaSorin S.p.A.
- Epitope Diagnostics, Inc.
- Exact Sciences Corporation
- Genova Diagnostics
- Hologic, Inc.
- Meridian Bioscience, Inc.
- Nanosphere, Inc.
- Quest Diagnostics
- Roche Diagnostics
- ScheBo Biotech AG
- Siemens Healthineers
- Trinity Biotech
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 576 |
| Published | January 2026 |
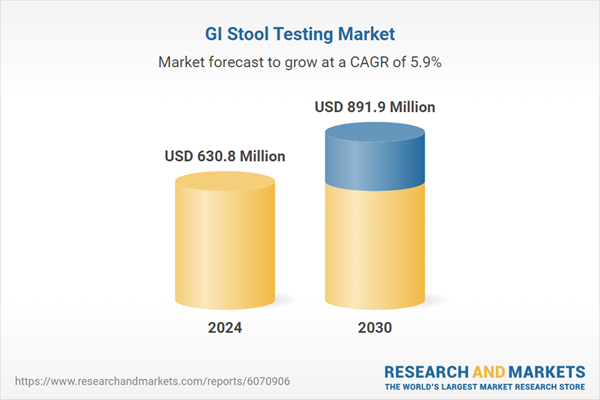
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 630.8 Million |
| Forecasted Market Value ( USD | $ 891.9 Million |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | Global |