Pharmacogenomics Technology Market: Key Trends & Drivers Summarized
How Is Pharmacogenomics Transforming Drug Development and Personalized Medicine?
Pharmacogenomics technology is revolutionizing the pharmaceutical industry by enabling personalized medicine based on an individual's genetic makeup. By studying how genetic variations influence drug response, pharmacogenomics allows for the development of targeted therapies, optimized drug dosages, and reduced adverse reactions. This shift from a one-size-fits-all approach to precision medicine enhances treatment efficacy and minimizes trial-and-error prescribing, significantly improving patient outcomes.Regulatory agencies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and the International Council for Harmonisation (ICH) are increasingly recognizing pharmacogenomics in drug approvals, labeling, and post-market surveillance. Many pharmaceutical companies are integrating pharmacogenomic data into clinical trials to identify biomarkers, stratify patient populations, and accelerate drug development timelines. With the rapid advancement of next-generation sequencing (NGS), CRISPR-based gene editing, and artificial intelligence (AI)-powered genetic analysis, pharmacogenomics is poised to become a cornerstone of precision medicine.
What Are the Key Trends Driving the Growth of Pharmacogenomics Technology?
The field of pharmacogenomics is evolving rapidly, driven by breakthroughs in genomic sequencing, bioinformatics, and AI-driven drug discovery. One of the most significant trends is the widespread adoption of whole-genome sequencing (WGS) and next-generation sequencing (NGS), which enable rapid and cost-effective identification of genetic variants linked to drug metabolism. With the decreasing cost of sequencing technologies, pharmacogenomics testing is becoming more accessible for clinical applications and population-wide studies.Another key trend is the integration of AI and machine learning (ML) in pharmacogenomics research. AI-powered algorithms can analyze vast amounts of genetic data to identify drug-gene interactions, predict treatment responses, and develop personalized drug regimens. AI-driven drug discovery platforms are also accelerating the identification of novel biomarkers and therapeutic targets, reducing the time and cost associated with traditional drug development.
The rise of direct-to-consumer (DTC) pharmacogenomics testing is another transformative trend. Companies like 23andMe and Color Genomics are offering genetic testing kits that provide consumers with insights into their drug metabolism and potential adverse drug reactions. While regulatory oversight remains a challenge, the increasing demand for consumer-driven healthcare is fueling the growth of this market.
Furthermore, CRISPR-based gene editing and gene therapy advancements are expanding the scope of pharmacogenomics beyond diagnostics into therapeutic applications. Gene-editing technologies are enabling customized drug development, targeted cancer therapies, and rare disease treatments, further propelling the demand for pharmacogenomics-driven precision medicine.
How Are End-Use Applications Shaping the Pharmacogenomics Market?
Pharmacogenomics technology is influencing multiple therapeutic areas, including oncology, cardiology, psychiatry, neurology, and infectious diseases. Each segment benefits from pharmacogenomics in unique ways, optimizing drug selection and treatment strategies.Oncology is one of the most significant areas where pharmacogenomics is making an impact. Targeted cancer therapies such as HER2 inhibitors for breast cancer (trastuzumab) and EGFR inhibitors for lung cancer (gefitinib) are based on genetic profiling. Pharmacogenomic testing helps oncologists determine which patients will benefit most from specific treatments, reducing toxicity and improving survival rates. The rise of companion diagnostics, which pair genetic testing with specific cancer drugs, is further driving growth in this sector.
In cardiology, pharmacogenomics is being used to tailor treatments for hypertension, anticoagulation, and cholesterol management. Genetic testing can predict how patients metabolize clopidogrel (Plavix), warfarin, and statins, ensuring that they receive the most effective and safest dosage. This reduces the risk of adverse drug reactions (ADRs) and thrombotic complications, improving cardiovascular disease management.
Psychiatry and neurology are also experiencing significant advancements due to pharmacogenomics. Antidepressants, antipsychotics, and epilepsy medications often exhibit high variability in patient response. Genetic testing helps predict how individuals metabolize drugs such as SSRIs, lithium, and carbamazepine, reducing treatment resistance and side effects. Pharmacogenomics is also playing a role in Alzheimer's and Parkinson's disease research, helping to identify potential therapeutic targets based on genetic risk factors.
In the infectious disease sector, pharmacogenomics is being applied to antiviral therapies, antibiotic resistance studies, and vaccine development. For example, genetic testing can determine how patients respond to HIV treatments like abacavir (HLA-B*5701 screening) or hepatitis C therapies, improving antiviral efficacy and reducing hypersensitivity reactions.
Additionally, pharmacogenomics is playing a crucial role in rare disease drug development and orphan drug approvals. Many genetic disorders require highly targeted therapies, and pharmacogenomics is enabling the identification of gene-based treatment strategies for conditions like cystic fibrosis, sickle cell disease, and Duchenne muscular dystrophy.
What Factors Are Driving the Growth of the Pharmacogenomics Technology Market?
The growth in the pharmacogenomics technology market is driven by several factors, including advancements in genomic sequencing, increasing demand for personalized medicine, regulatory support, and expanding applications in drug development. As healthcare shifts toward precision medicine, pharmaceutical companies, research institutions, and healthcare providers are investing heavily in genetic testing, bioinformatics platforms, and AI-driven drug discovery tools.One of the primary growth drivers is the decreasing cost of genetic sequencing. The cost of whole-genome sequencing has dropped significantly, making pharmacogenomics testing more accessible and cost-effective. As a result, healthcare providers are incorporating genetic profiling into routine clinical practice, expanding the market for pharmacogenomics-based diagnostics and therapeutics.
Regulatory agencies are also playing a key role in promoting pharmacogenomics. The FDA has issued pharmacogenomic labeling for over 300 drugs, highlighting genetic markers that influence drug metabolism and efficacy. Additionally, initiatives such as the All of Us Research Program (U.S.) and the UK Biobank Project are collecting large-scale genetic data to advance pharmacogenomics research, fueling further adoption of the technology.
The increasing prevalence of chronic diseases, cancer, and drug-resistant infections is also driving demand for pharmacogenomics solutions. As traditional treatment approaches often result in variable drug responses and adverse reactions, pharmacogenomics provides a solution by enabling precision prescribing and individualized treatment strategies.& Furthermore, pharmaceutical companies are leveraging pharmacogenomics to optimize clinical trials and accelerate drug development. By stratifying patient populations based on genetic profiles, companies can improve trial success rates, reduce drug development costs, and bring therapies to market faster. The rise of companion diagnostics and biomarker-driven drug approvals is further strengthening the link between pharmacogenomics and pharmaceutical innovation.
Additionally, government funding and public-private partnerships are fueling research and commercialization efforts. Organizations such as the National Institutes of Health (NIH), the European Medicines Agency (EMA), and the Japan Agency for Medical Research and Development (AMED) are investing in pharmacogenomics research, enabling breakthroughs in gene-based therapy development and population-wide genetic screening initiatives.& As pharmacogenomics continues to integrate with AI, big data, and precision drug discovery, the field is poised for exponential growth, transforming the way drugs are developed, prescribed, and monitored. With advancements in gene sequencing, biomarker identification, and AI-driven analysis, pharmacogenomics will play an increasingly central role in shaping the future of personalized medicine and precision healthcare.
Report Scope
The report analyzes the Pharmacogenomics Technology market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Therapeutic Area (Oncology, Neurological Disorders, Cardiovascular Disease, Immunological Disorders, Infectious Diseases); Technology (PCR, In-situ Hybridization, Immunohistochemistry, Sequencing, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Oncology Therapeutic Area segment, which is expected to reach US$5.3 Billion by 2030 with a CAGR of a 9.4%. The Neurological Disorders Therapeutic Area segment is also set to grow at 8.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.9 Billion in 2024, and China, forecasted to grow at an impressive 12.9% CAGR to reach $2.5 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pharmacogenomics Technology Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pharmacogenomics Technology Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pharmacogenomics Technology Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 23andMe, Inc., Abbott Laboratories, Agilent Technologies, Inc., Becton, Dickinson and Company, bioMérieux S.A. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Pharmacogenomics Technology market report include:
- 23andMe, Inc.
- Abbott Laboratories
- Agilent Technologies, Inc.
- Becton, Dickinson and Company
- bioMérieux S.A.
- Bio-Rad Laboratories, Inc.
- Coriell Life Sciences
- Eurofins Scientific
- F. Hoffmann-La Roche Ltd
- Genomind, Inc.
- Guardant Health, Inc.
- Illumina, Inc.
- Merck KGaA
- Myriad Genetics, Inc.
- Pacific Biosciences of California, Inc.
- Pathway Genomics Corporation
- PerkinElmer, Inc.
- QIAGEN N.V.
- Thermo Fisher Scientific Inc.
- Wellness Labs
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 23andMe, Inc.
- Abbott Laboratories
- Agilent Technologies, Inc.
- Becton, Dickinson and Company
- bioMérieux S.A.
- Bio-Rad Laboratories, Inc.
- Coriell Life Sciences
- Eurofins Scientific
- F. Hoffmann-La Roche Ltd
- Genomind, Inc.
- Guardant Health, Inc.
- Illumina, Inc.
- Merck KGaA
- Myriad Genetics, Inc.
- Pacific Biosciences of California, Inc.
- Pathway Genomics Corporation
- PerkinElmer, Inc.
- QIAGEN N.V.
- Thermo Fisher Scientific Inc.
- Wellness Labs
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 291 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
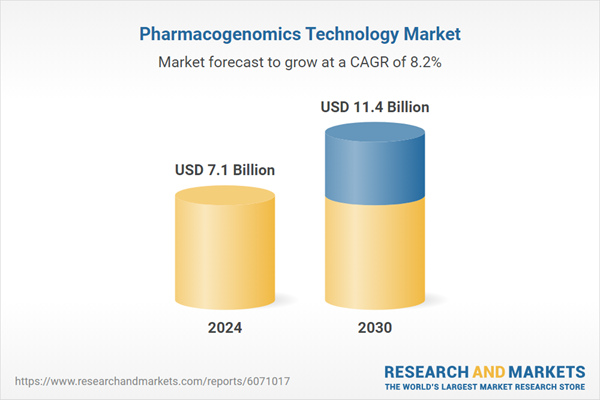
| Estimated Market Value ( USD | $ 7.1 Billion |
| Forecasted Market Value ( USD | $ 11.4 Billion |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | Global |