Global Mucosal Atomization Device Market - Key Trends & Drivers Summarized
Why Are Mucosal Atomization Devices Becoming Essential in Drug Delivery?
Mucosal atomization devices have emerged as a critical tool in modern drug delivery, offering needle-free administration with enhanced absorption and rapid onset of action. These devices allow for the efficient and pain-free delivery of medications through the nasal, buccal, or oropharyngeal mucosa, making them particularly valuable in emergency medicine, pain management, and vaccine delivery. Their ability to provide systemic drug absorption without the need for injections has increased their popularity among healthcare providers and patients, reducing the risk of needle-stick injuries and improving patient compliance. The increasing prevalence of respiratory conditions, opioid overdoses, and anaphylactic emergencies has further fueled the demand for mucosal atomization devices, as they provide a quick and effective route for drug administration. Additionally, their role in pediatric and geriatric care, where needle aversion is a common concern, has solidified their place in modern medical practice. As pharmaceutical companies continue to explore innovative drug formulations suitable for mucosal delivery, these devices are set to play a greater role in healthcare.What Technological Advancements Are Improving Mucosal Atomization Devices?
Technological innovations have significantly enhanced the functionality and effectiveness of mucosal atomization devices, leading to improved drug absorption rates and precision in dose delivery. Advances in spray nozzle design have enabled the production of finer, more uniform mist particles, optimizing drug absorption across the mucosal membrane. The integration of electronic control mechanisms has facilitated the development of adjustable flow rate atomizers, allowing for tailored dosing based on patient needs. Additionally, smart atomization devices with connectivity features are being explored, providing healthcare professionals with real-time data tracking for adherence monitoring and personalized treatment plans. The use of biocompatible and eco-friendly materials in device manufacturing has further improved patient safety and regulatory compliance. With the ongoing development of combination therapies designed specifically for mucosal delivery, device manufacturers are working closely with pharmaceutical companies to ensure seamless drug-device compatibility. These advancements are not only improving drug efficacy but also expanding the application of mucosal atomization devices across multiple therapeutic areas.How Are Regulatory Approvals and Market Trends Shaping Mucosal Atomization Device Adoption?
Regulatory agencies worldwide are recognizing the benefits of mucosal atomization devices and have introduced guidelines to ensure their safety, efficacy, and standardization. The FDA and EMA have established stringent protocols for approving these devices, requiring robust clinical evidence demonstrating bioavailability, stability, and patient safety. The increasing number of regulatory approvals for intranasal medications, such as opioid antagonists and sedatives, has further driven the adoption of atomization devices in emergency care settings. Additionally, healthcare providers are increasingly favoring non-invasive drug delivery options to enhance patient comfort and reduce the burden of training associated with injectable medications. The rise of home healthcare and self-administration trends has also influenced the market, with manufacturers developing compact, easy-to-use atomization devices for at-home use. As governments and healthcare systems push for needle-free alternatives to improve safety and compliance, mucosal atomization devices are expected to witness accelerated growth in both clinical and consumer healthcare markets.What Are the Key Growth Drivers in the Mucosal Atomization Device Market?
The growth in the mucosal atomization device market is driven by several factors, including the increasing prevalence of chronic and emergency medical conditions, advancements in drug delivery technology, and the rising demand for non-invasive administration methods. The surge in opioid overdose cases has led to heightened demand for intranasal naloxone atomizers, which are now widely distributed across emergency response teams, pharmacies, and community health programs. Additionally, the growing adoption of intranasal vaccines and biologics, particularly in response to global pandemics, has created new opportunities for mucosal atomization device manufacturers. The expanding geriatric population and rising incidence of neurological disorders have also increased demand for rapid and effective drug delivery solutions that bypass gastrointestinal metabolism. The convenience and safety benefits of mucosal atomization devices have driven their adoption in hospital settings, ambulatory care, and at-home treatments. As pharmaceutical innovation continues to focus on enhancing drug delivery efficiency, the mucosal atomization device market is expected to experience sustained growth, offering critical solutions for both acute and long-term medical needs.Report Scope
The report analyzes the Mucosal Atomization Device market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product Type (Nasal Atomization Devices, Fiber Optic Atomization Devices, Laryngo Tracheal Atomization Devices, Bottle Atomizers); Technology (Gas Propelled Atomization Technology, Electrical Atomization Technology); End-Use (Hospitals End-Use, Ambulatory Surgery Centers End-Use, Specialty Clinics End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Nasal Atomization Devices segment, which is expected to reach US$457.1 Million by 2030 with a CAGR of a 4.9%. The Fiber Optic Atomization Devices segment is also set to grow at 4.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $202.2 Million in 2024, and China, forecasted to grow at an impressive 8.1% CAGR to reach $198.8 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Mucosal Atomization Device Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Mucosal Atomization Device Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Mucosal Atomization Device Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Aesculap Implant Systems, LLC, Amplitude Surgical, B. Braun Melsungen AG, Baumer S.A, ConforMIS, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Mucosal Atomization Device market report include:
- Becton, Dickinson and Company
- BTME Group Ltd. (Medtree)
- BVM Meditech Pvt. Ltd.
- Cook Medical, Inc.
- DeVilbiss Healthcare GmbH
- Drive DeVilbiss Healthcare
- Integra LifeSciences
- Intersurgical Ltd.
- Kurve Technology, Inc.
- Life-Assist Inc.
- Medica Holdings, LLC
- Medline Industries, LP
- Medspray BV
- Parker Laboratories, Inc.
- Pulmodyne, Inc.
- Salter Labs
- Smiths Medical
- Teleflex Incorporated
- Vyaire Medical
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Becton, Dickinson and Company
- BTME Group Ltd. (Medtree)
- BVM Meditech Pvt. Ltd.
- Cook Medical, Inc.
- DeVilbiss Healthcare GmbH
- Drive DeVilbiss Healthcare
- Integra LifeSciences
- Intersurgical Ltd.
- Kurve Technology, Inc.
- Life-Assist Inc.
- Medica Holdings, LLC
- Medline Industries, LP
- Medspray BV
- Parker Laboratories, Inc.
- Pulmodyne, Inc.
- Salter Labs
- Smiths Medical
- Teleflex Incorporated
- Vyaire Medical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 367 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
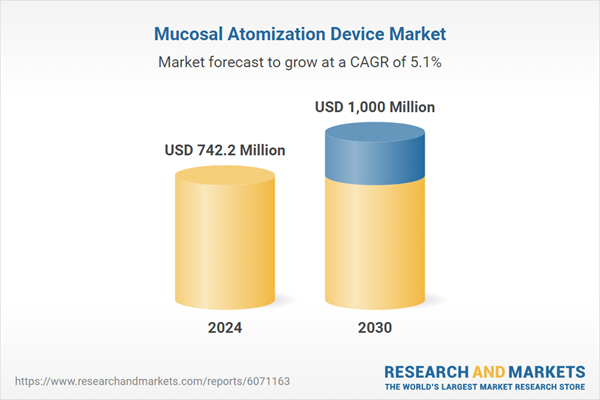
| Estimated Market Value ( USD | $ 742.2 Million |
| Forecasted Market Value ( USD | $ 1000 Million |
| Compound Annual Growth Rate | 5.1% |
| Regions Covered | Global |