Global Bioequivalence Studies Market - Key Trends & Drivers Summarized
Why Are Bioequivalence Studies Critical in Generic Drug Development?
Bioequivalence studies play an essential role in the pharmaceutical industry, particularly in the development and approval of generic drugs. These studies evaluate whether a generic version of a drug demonstrates the same pharmacokinetic properties - absorption, distribution, metabolism, and excretion - as its branded counterpart. Regulatory agencies such as the U.S. FDA, European Medicines Agency (EMA), and other global health authorities mandate bioequivalence studies to ensure that generic medications provide the same therapeutic effect, safety profile, and bioavailability as innovator drugs. With the growing emphasis on cost-effective healthcare and the increasing expiration of patents on blockbuster drugs, pharmaceutical companies are investing heavily in bioequivalence testing to bring high-quality generics to market. Additionally, advancements in analytical technologies and computational modeling are improving the precision and efficiency of bioequivalence studies, facilitating faster regulatory approvals and market entry for generic drug manufacturers. As healthcare systems worldwide seek affordable treatment alternatives, bioequivalence studies remain a cornerstone of pharmaceutical innovation and accessibility.How Are Technological Advancements Enhancing the Efficiency of Bioequivalence Testing?
Significant technological advancements in pharmacokinetics and clinical research are revolutionizing bioequivalence studies, improving their accuracy, efficiency, and cost-effectiveness. The integration of advanced liquid chromatography-mass spectrometry (LC-MS) techniques has enhanced the sensitivity and precision of drug concentration measurements, allowing for more robust bioavailability assessments. Additionally, the adoption of physiologically based pharmacokinetic (PBPK) modeling and in silico simulations is reducing the reliance on human clinical trials, streamlining drug development timelines while maintaining regulatory compliance. Another breakthrough in bioequivalence research is the application of real-time data analytics and machine learning algorithms, enabling early prediction of pharmacokinetic behavior and identifying potential variability between formulations. Moreover, innovations in biosimilar bioequivalence studies are facilitating the approval of complex biologic drugs, expanding the scope of bioequivalence beyond traditional small-molecule generics. With continuous advancements in study methodologies, bioequivalence testing is becoming faster, more reliable, and more adaptable to evolving regulatory frameworks, ensuring that generic and biosimilar drugs meet the highest standards of safety and efficacy.What Market Trends Are Driving the Growth of Bioequivalence Studies?
Several key market trends are influencing the expansion of bioequivalence studies, reflecting the growing demand for affordable pharmaceuticals and regulatory harmonization efforts. The increasing global acceptance of generics as a cost-effective alternative to branded drugs has led to a surge in bioequivalence testing requirements, particularly in emerging markets. Regulatory agencies are standardizing bioequivalence guidelines across multiple jurisdictions, allowing for simultaneous drug approvals in multiple regions and reducing market entry barriers for pharmaceutical companies. Additionally, the rise of complex generics, including inhalation therapies, ophthalmic solutions, and transdermal patches, is driving the need for more sophisticated bioequivalence study designs. The rapid expansion of contract research organizations (CROs) specializing in bioequivalence testing is another prominent trend, as pharmaceutical firms seek to outsource clinical trials to reduce operational costs and focus on core drug development. Furthermore, the growing adoption of decentralized clinical trials (DCTs) and digital health technologies is modernizing bioequivalence research, improving patient recruitment, compliance, and real-world data collection. As the global pharmaceutical industry continues to evolve, bioequivalence studies will remain a fundamental component of drug approval and market expansion strategies.What Are the Key Factors Fueling the Growth of the Bioequivalence Studies Market?
The growth in the bioequivalence studies market is driven by several factors, including the rising demand for generic drugs, increasing regulatory scrutiny, and advancements in clinical trial methodologies. The expiration of patents on high-revenue pharmaceutical products is creating a lucrative opportunity for generic drug manufacturers, necessitating comprehensive bioequivalence studies to ensure market approval. Additionally, regulatory agencies are imposing stricter compliance requirements on bioequivalence data, prompting pharmaceutical companies to adopt advanced testing methodologies and invest in high-precision analytical tools. The outsourcing of bioequivalence studies to CROs is also accelerating market growth, allowing pharmaceutical firms to expedite drug development while optimizing resources. The expanding biosimilars market, driven by the need for cost-effective biologic therapies, is further fueling demand for bioequivalence studies tailored to large-molecule drugs. Moreover, emerging markets such as India, China, and Brazil are witnessing a surge in bioequivalence testing due to their growing generic pharmaceutical industries and regulatory alignment with international standards. As healthcare systems worldwide strive to balance affordability and innovation, bioequivalence studies will continue to be a vital enabler of pharmaceutical accessibility and cost reduction.Report Scope
The report analyzes the Bioequivalence Studies market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Molecule Type (Small Molecule Bioequivalence Studies, Large Molecule Bioequivalence Studies); Dosage Form (Solid Oral Dosage, Parenteral Formulations, Topical Products, Other Dosage Forms); Therapeutic Area (Oncology Therapeutic Area, Neurology Therapeutic Area, Immunology Therapeutic Area, Metabolic Disorders Therapeutic Area, Hematology Therapeutic Area, Other Therapeutic Areas).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Small Molecule Bioequivalence Studies segment, which is expected to reach US$615.3 Million by 2030 with a CAGR of a 8.3%. The Large Molecule Bioequivalence Studies segment is also set to grow at 4.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $206.9 Million in 2024, and China, forecasted to grow at an impressive 10.8% CAGR to reach $235.5 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Bioequivalence Studies Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Bioequivalence Studies Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Bioequivalence Studies Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Anhui Ancheng Bamboo and Wood Tableware Co., Ltd., BETTER EARTH LLC, Better Earth Packaging, BioPak Pty Ltd, BIOTREM SP. Z O.O. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Bioequivalence Studies market report include:
- Charles River Laboratories International, Inc.
- Geropharm
- ICON plc
- Inhibikase Therapeutics
- Intertek Group Plc
- IQVIA, Inc.
- KYMOS Group
- Labcorp Drug Development
- Lupin Ltd.
- Malvern Panalytical Ltd.
- NorthEast BioAnalytical Laboratories LLC
- Novo Nordisk A/S
- Novotech
- SGS SA
- Syneos Health
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Charles River Laboratories International, Inc.
- Geropharm
- ICON plc
- Inhibikase Therapeutics
- Intertek Group Plc
- IQVIA, Inc.
- KYMOS Group
- Labcorp Drug Development
- Lupin Ltd.
- Malvern Panalytical Ltd.
- NorthEast BioAnalytical Laboratories LLC
- Novo Nordisk A/S
- Novotech
- SGS SA
- Syneos Health
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 385 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
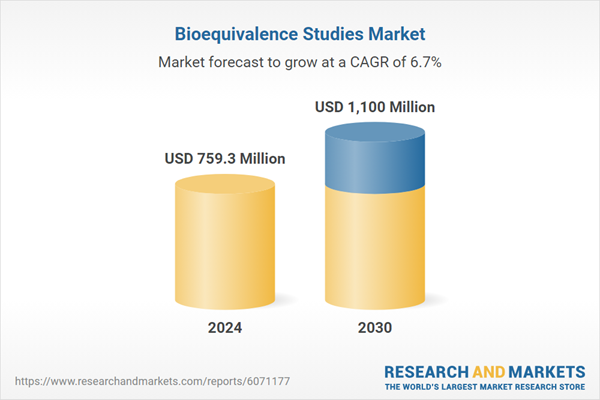
| Estimated Market Value ( USD | $ 759.3 Million |
| Forecasted Market Value ( USD | $ 1100 Million |
| Compound Annual Growth Rate | 6.7% |
| Regions Covered | Global |