Global Biologics Contract Development Market - Key Trends & Drivers Summarized
Why Is Biologics Contract Development Gaining Momentum in the Biopharmaceutical Industry?
Biologics contract development has become an essential part of the pharmaceutical ecosystem, enabling biotech firms and large pharmaceutical companies to accelerate drug discovery, streamline product development, and optimize manufacturing processes. With the increasing complexity of biologic drugs such as monoclonal antibodies, gene therapies, and recombinant proteins, companies are increasingly outsourcing key stages of biologics development to specialized contract development organizations (CDOs) that offer expertise, regulatory compliance, and advanced manufacturing capabilities. The demand for biologics is surging due to their effectiveness in treating chronic diseases such as cancer, autoimmune disorders, and rare genetic conditions. However, the high costs and technical complexities associated with biologic drug development pose significant challenges for in-house research and development teams. As a result, pharmaceutical companies are leveraging the expertise of CDOs to conduct early-stage research, cell line development, process optimization, and analytical testing, thereby reducing costs and accelerating time-to-market. With the rise of biosimilars and personalized medicine, the need for efficient contract development services is expected to grow substantially in the coming years.How Are Technological Advancements Enhancing Biologics Contract Development?
The integration of cutting-edge technologies such as artificial intelligence (AI), automation, and single-use bioprocessing systems is transforming biologics contract development, improving efficiency, scalability, and quality assurance. AI-driven drug discovery platforms are enabling faster identification of therapeutic candidates by analyzing vast datasets and predicting molecular interactions with high accuracy. This accelerates the initial stages of biologic development and reduces the time required for preclinical research. Additionally, the adoption of single-use bioreactors and modular production facilities is enhancing the flexibility and cost-effectiveness of biologic manufacturing. These innovations allow contract development organizations to quickly adapt to changing client requirements and improve production efficiency for small-batch and personalized biologic drugs. Moreover, advancements in cell line engineering and gene editing techniques such as CRISPR are improving the productivity and stability of biologic drug candidates, ensuring higher yields and improved therapeutic efficacy. As biologic therapies become more specialized and complex, technological innovation will continue to drive the evolution of contract development services.What Market Trends Are Driving the Growth of Biologics Contract Development?
One of the most significant trends shaping the biologics contract development market is the increasing demand for biosimilars and novel biologic therapies. As patents for blockbuster biologics expire, pharmaceutical companies are focusing on developing biosimilars to capture market share and provide more affordable treatment options. Contract development organizations play a crucial role in facilitating biosimilar development by providing expertise in analytical characterization, bioequivalence testing, and regulatory compliance. Another key trend is the shift toward personalized medicine and cell and gene therapy development. With advancements in genomic research and precision medicine, pharmaceutical companies are investing in biologic therapies tailored to individual patients. This shift is driving the demand for contract development services that specialize in advanced cell therapies, viral vector production, and customized biologic formulations. Additionally, regulatory agencies are introducing streamlined approval pathways for innovative biologics, encouraging companies to accelerate their development timelines through strategic outsourcing partnerships with contract development organizations.What Are the Key Growth Drivers for the Biologics Contract Development Market?
The growth in the Biologics Contract Development market is driven by several factors, including increasing demand for biologic drugs, rising investment in biosimilars, and the growing complexity of biologic manufacturing. As pharmaceutical companies seek to optimize costs and accelerate drug development timelines, contract development organizations are playing an increasingly critical role in the industry. The expansion of biologics manufacturing infrastructure, particularly in emerging markets, is further propelling market growth by providing cost-effective development solutions to biotech firms and small pharmaceutical companies. Additionally, the increasing adoption of automation, AI, and advanced bioprocessing technologies is enhancing the efficiency and scalability of biologics contract development services. As regulatory agencies continue to support the expansion of biologics and biosimilars, contract development organizations are expected to see sustained growth, reinforcing their position as key partners in the future of biopharmaceutical innovation.Report Scope
The report analyzes the Biologics Contract Development market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Source (Mammalian Source, Microbial Source, Other Sources); Service (Process Development Service, Cell Line Development Service, Other Services); Indication (Oncology Indication, Immunological Disorders Indication, Cardiovascular Disorders Indication, Hematological Disorders Indication, Other Indications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Mammalian Source segment, which is expected to reach US$6.6 Billion by 2030 with a CAGR of a 7.2%. The Microbial Source segment is also set to grow at 7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.4 Billion in 2024, and China, forecasted to grow at an impressive 7.3% CAGR to reach $2.2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Biologics Contract Development Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Biologics Contract Development Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Biologics Contract Development Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Aroa Biosurgery Limited, AVITA Medical, Inc., Integra LifeSciences Holdings Corporation, Mallinckrodt Pharmaceuticals plc, MIMEDX Group, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Biologics Contract Development market report include:
- Biovian Oy
- Boehringer Ingelheim International GmbH
- Catalent, Inc.
- Celonic AG
- Charles River Laboratories
- FUJIFILM Corporation
- Kemwell Biopharma Pvt. Ltd.
- Lonza Group
- LOTTE BIOLOGICS
- Mabxience Holding S.L.
- Polymun Scientific Immunbiologische Forschung GmbH
- Samsung Biologics Co., Ltd.
- Scorpius BioManufacturing
- Syngene International Ltd.
- WuXi Biologics
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Biovian Oy
- Boehringer Ingelheim International GmbH
- Catalent, Inc.
- Celonic AG
- Charles River Laboratories
- FUJIFILM Corporation
- Kemwell Biopharma Pvt. Ltd.
- Lonza Group
- LOTTE BIOLOGICS
- Mabxience Holding S.L.
- Polymun Scientific Immunbiologische Forschung GmbH
- Samsung Biologics Co., Ltd.
- Scorpius BioManufacturing
- Syngene International Ltd.
- WuXi Biologics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 172 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
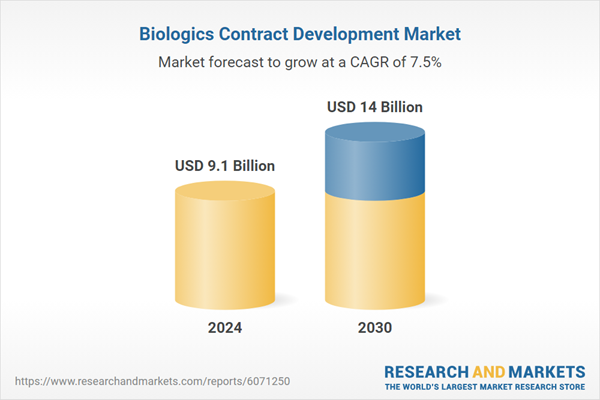
| Estimated Market Value ( USD | $ 9.1 Billion |
| Forecasted Market Value ( USD | $ 14 Billion |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | Global |