Global Pneumococcal Vaccine Market - Key Trends & Drivers Summarized
Why Is the Pneumococcal Vaccine Critical for Public Health?
Pneumococcal vaccines are essential in preventing infections caused by Streptococcus pneumoniae, a bacterium responsible for serious diseases such as pneumonia, meningitis, and sepsis. These infections pose a significant health risk, particularly to infants, young children, elderly individuals, and immunocompromised patients. The widespread adoption of pneumococcal vaccines has led to a substantial reduction in pneumococcal-related mortality and morbidity worldwide, reinforcing their importance in global immunization programs.Governments and healthcare organizations, including the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC), strongly advocate for routine pneumococcal vaccination to protect vulnerable populations. National immunization programs in high-income and developing countries have accelerated the uptake of pneumococcal conjugate vaccines (PCVs) and pneumococcal polysaccharide vaccines (PPSVs), significantly lowering infection rates. Additionally, the COVID-19 pandemic heightened awareness of respiratory infections, leading to increased demand for pneumococcal vaccination as part of broader public health initiatives to reduce complications from bacterial pneumonia in COVID-19 patients.
How Are Innovations in Vaccine Development Transforming the Market?
The pneumococcal vaccine market has evolved rapidly due to advancements in biotechnology, vaccine formulations, and immunization strategies. One of the most significant innovations is the development of next-generation pneumococcal conjugate vaccines (PCVs) with broader serotype coverage. Leading vaccine manufacturers, including Pfizer, Merck, and GlaxoSmithKline (GSK), have introduced multi-valent PCVs that offer protection against an expanded range of pneumococcal strains, addressing the challenge of emerging antibiotic-resistant and non-vaccine serotypes.For instance, Pfizer's PREVNAR 20 and Merck's VAXNEUVANCE (PCV15) are next-generation vaccines designed to provide enhanced immunity against pneumococcal strains responsible for severe infections. These vaccines represent a major advancement over older formulations such as PREVNAR 13 and PCV10, which offered protection against fewer serotypes. Additionally, research into protein-based pneumococcal vaccines is gaining momentum, aiming to provide broader and more durable immunity against diverse pneumococcal strains.
Another transformative development in the market is the integration of mRNA vaccine technology in pneumococcal vaccine research. Inspired by the success of mRNA COVID-19 vaccines, companies are exploring mRNA-based pneumococcal vaccines that could offer quicker development timelines, improved efficacy, and adaptability against emerging serotypes. Such innovations have the potential to revolutionize pneumococcal disease prevention, especially in regions with high disease burdens.
Why Is Global Demand for Pneumococcal Vaccination Increasing?
The demand for pneumococcal vaccines is rising globally due to expanding immunization programs, aging populations, and increased awareness of respiratory infections. Countries with high infant mortality rates and a high burden of pneumococcal disease, such as those in Africa, South Asia, and Latin America, are scaling up vaccination efforts through partnerships with organizations like Gavi, the Vaccine Alliance, and the Global Pneumococcal Vaccination Initiative. These initiatives aim to improve vaccine access in low-income regions, significantly driving market expansion.The aging global population is another critical factor fueling demand for pneumococcal vaccination. Older adults, especially those with underlying health conditions such as diabetes, chronic obstructive pulmonary disease (COPD), and cardiovascular diseases, face a heightened risk of pneumococcal infections. As a result, many countries have introduced adult pneumococcal vaccination programs, encouraging widespread immunization beyond childhood schedules. The rising prevalence of antimicrobial resistance (AMR) has further emphasized the need for preventive vaccination, as drug-resistant pneumococcal strains limit treatment options, making vaccination the most effective defense.
Additionally, the post-pandemic focus on respiratory health has accelerated pneumococcal vaccine uptake. Many public health agencies now recommend pneumococcal vaccination alongside flu and COVID-19 boosters for high-risk populations, integrating it into routine adult immunization programs. The pharmaceutical industry's efforts to simplify dosing schedules and develop single-dose combination vaccines (e.g., pneumococcal vaccines combined with influenza or RSV vaccines) are expected to further drive uptake, particularly among elderly patients and immunocompromised individuals.
What Factors Are Driving the Growth of the Pneumococcal Vaccine Market?
The growth in the pneumococcal vaccine market is driven by several factors, including technological advancements, increasing global immunization coverage, and rising healthcare investments. One of the primary drivers is the expansion of pediatric and adult vaccination programs, supported by government policies and international funding initiatives. Countries are incorporating pneumococcal vaccines into routine immunization schedules, contributing to widespread market growth.Another key factor is pharmaceutical innovation and competitive product development. Vaccine manufacturers are continuously enhancing their formulations to provide broader protection against evolving pneumococcal strains. The launch of multi-valent PCVs and combination vaccines is expected to shape the future of the market, offering longer-lasting immunity and reduced booster dose requirements. Additionally, investments in cold chain infrastructure and vaccine distribution networks are improving access to pneumococcal vaccines in remote and underserved regions.
The rising burden of pneumococcal diseases in emerging economies is also driving demand. Governments in countries like India, China, and Brazil are increasingly prioritizing pneumococcal vaccination as part of their public health strategies, leading to higher adoption rates. Additionally, corporate partnerships and public-private collaborations are helping reduce vaccine costs, making immunization more affordable for lower-income populations.
The growth of travel and medical tourism is another factor influencing the market. Many travelers, particularly those visiting regions with high pneumococcal disease prevalence, are opting for preventive vaccination, increasing demand in both developed and developing countries.
With ongoing innovations in vaccine technology, increasing awareness of pneumococcal disease prevention, and strong government and private sector support, the pneumococcal vaccine market is poised for steady expansion. As global healthcare priorities continue to shift toward preventive care and immunization, pneumococcal vaccines will remain a cornerstone of infectious disease prevention worldwide.
Report Scope
The report analyzes the Pneumococcal Vaccines market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Vaccine Type (Pneumococcal Conjugate Vaccine, Pneumococcal Polysaccharide Vaccine); Product Type (Prevnar 13, Synflorix, Pneumovax 23, Vaxneuvance, Pneumosil, Other Product Types).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Pneumococcal Conjugate Vaccine segment, which is expected to reach US$7.4 Billion by 2030 with a CAGR of a 6.2%. The Pneumococcal Polysaccharide Vaccine segment is also set to grow at 3.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.3 Billion in 2024, and China, forecasted to grow at an impressive 8.5% CAGR to reach $2.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pneumococcal Vaccines Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pneumococcal Vaccines Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pneumococcal Vaccines Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AIM Vaccine Co., Ltd., AstraZeneca, Beijing Minhai Biotechnology Co., Ltd., Bharat Biotech International Ltd., Biological E. Limited and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Pneumococcal Vaccines market report include:
- AIM Vaccine Co., Ltd.
- AstraZeneca
- Beijing Minhai Biotechnology Co., Ltd.
- Bharat Biotech International Ltd.
- Biological E. Limited
- CSL Limited
- Daiichi Sankyo Company, Limited
- Emergent BioSolutions Inc.
- GlaxoSmithKline plc (GSK)
- Merck & Co., Inc.
- Mitsubishi Tanabe Pharma Corporation
- Novartis International AG
- Panacea Biotec Ltd.
- Pfizer Inc.
- Sanofi
- Serum Institute of India Pvt. Ltd.
- Sinopharm Group Co., Ltd.
- Sinovac Biotech Ltd.
- Vaxcyte, Inc.
- Walvax Biotechnology Co., Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AIM Vaccine Co., Ltd.
- AstraZeneca
- Beijing Minhai Biotechnology Co., Ltd.
- Bharat Biotech International Ltd.
- Biological E. Limited
- CSL Limited
- Daiichi Sankyo Company, Limited
- Emergent BioSolutions Inc.
- GlaxoSmithKline plc (GSK)
- Merck & Co., Inc.
- Mitsubishi Tanabe Pharma Corporation
- Novartis International AG
- Panacea Biotec Ltd.
- Pfizer Inc.
- Sanofi
- Serum Institute of India Pvt. Ltd.
- Sinopharm Group Co., Ltd.
- Sinovac Biotech Ltd.
- Vaxcyte, Inc.
- Walvax Biotechnology Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 285 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
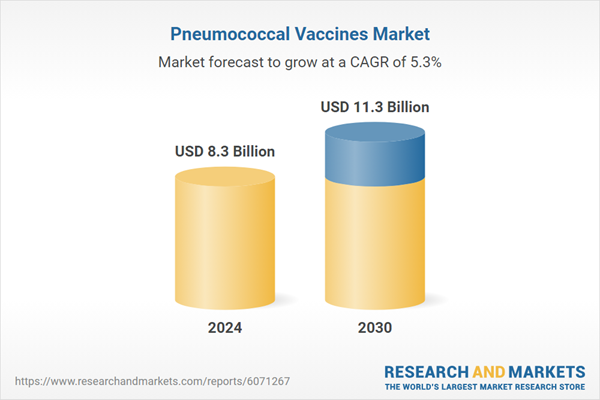
| Estimated Market Value ( USD | $ 8.3 Billion |
| Forecasted Market Value ( USD | $ 11.3 Billion |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |