Global Clinical Trial Investigative Site Network Market - Key Trends & Drivers Summarized
Why Are Investigative Site Networks Critical to Clinical Trials?
Investigative site networks (ISNs) play a pivotal role in streamlining clinical trials by providing a structured and well-coordinated approach to site selection, patient enrollment, and study execution. These networks consist of pre-qualified, research-ready clinical trial sites that adhere to standardized protocols, regulatory requirements, and data collection methodologies. The increasing complexity of clinical research, particularly in oncology, rare diseases, and personalized medicine, has heightened the demand for efficient and well-managed site networks. Investigative site networks help pharmaceutical companies, contract research organizations (CROs), and sponsors accelerate study timelines by reducing site activation delays and improving patient recruitment. Moreover, ISNs ensure consistency in trial execution, facilitating better compliance with regulatory guidelines and minimizing variability in study data. With the growing adoption of decentralized and hybrid clinical trials, the role of ISNs is expanding to include remote patient monitoring and virtual trial capabilities. As clinical trials become more globalized, investigative site networks are enhancing operational efficiency, reducing costs, and improving overall trial success rates.How Are Technology and Data Analytics Enhancing ISN Efficiency?
Advancements in technology and data analytics are significantly improving the efficiency of investigative site networks by optimizing trial site selection, patient recruitment, and protocol adherence. Artificial intelligence (AI) and predictive analytics are enabling real-time performance monitoring of clinical trial sites, helping sponsors identify high-performing sites based on historical recruitment rates, protocol compliance, and patient retention metrics. Electronic data capture (EDC) systems and cloud-based site management platforms are streamlining workflows, reducing administrative burdens, and ensuring faster regulatory submissions. Blockchain technology is being explored to enhance data security, ensuring the integrity of trial results across multiple study sites. Additionally, machine learning algorithms are being employed to match trial sites with suitable patient populations, improving enrollment rates and reducing dropout risks. The integration of wearable technology and remote monitoring solutions within ISNs is further expanding the scope of clinical trials by allowing real-world data collection from diverse patient populations.What Are the Key Trends Driving Investigative Site Networks?
Several key trends are shaping the growth and evolution of investigative site networks in clinical research. The increasing adoption of virtual and decentralized trials is driving the need for hybrid site networks that can support both in-person and remote patient interactions. The rise of patient-centric trial designs is prompting ISNs to invest in digital health solutions, telemedicine platforms, and mobile health (mHealth) applications to improve patient engagement and adherence. The globalization of clinical trials is also fueling demand for geographically diverse site networks that can facilitate multi-regional studies and expand patient access to innovative therapies. Additionally, the growing focus on diversity and inclusion in clinical trials is leading ISNs to establish partnerships with community-based research centers and underserved populations. The push toward faster drug development timelines is encouraging pharmaceutical companies and CROs to rely more heavily on ISNs to accelerate site activation, standardize trial procedures, and enhance regulatory compliance. These emerging trends are positioning investigative site networks as indispensable partners in the evolving landscape of clinical trials.What’ s Driving the Growth of the Clinical Trial Investigative Site Network Market?
The growth in the clinical trial investigative site network market is driven by several factors, including the increasing complexity of clinical trials, the need for faster patient recruitment, and technological advancements in trial site management. The rise of personalized medicine and targeted therapies is necessitating the use of specialized trial sites with expertise in genomics, rare diseases, and immunotherapies. Regulatory pressures to ensure data integrity and patient safety are driving the adoption of standardized site networks that adhere to Good Clinical Practice (GCP) guidelines. The expansion of artificial intelligence (AI) and machine learning applications in trial site selection and performance analysis is further accelerating market growth. Additionally, the increasing adoption of remote patient monitoring and wearable health technologies is reshaping the role of ISNs, enabling trials to extend beyond traditional hospital-based settings. Strategic collaborations between ISNs, pharmaceutical companies, and CROs are fostering innovation and efficiency in clinical research, further propelling market expansion.Report Scope
The report analyzes the Clinical Trial Investigative Site Network market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Phase Type (Phase I, Phase II, Phase III, Phase IV); Therapeutic Areas (Oncology, Cardiology, CNS Conditions, Pain Management, Endocrine, Other Therapeutic Areas); End-Use (Pharmaceutical and Biopharmaceutical Companies End-Use, Medical Device Companies End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Phase I Clinical Trial segment, which is expected to reach US$5.9 Billion by 2030 with a CAGR of a 7.7%. The Phase II Clinical Trial segment is also set to grow at 6.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.3 Billion in 2024, and China, forecasted to grow at an impressive 10.7% CAGR to reach $2.6 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Clinical Trial Investigative Site Network Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Clinical Trial Investigative Site Network Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Clinical Trial Investigative Site Network Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Ancillare, LP, Bionical Emas, Bio-Rad Laboratories, Inc., Caligor Coghlan, ClientPharma and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Clinical Trial Investigative Site Network market report include:
- CenExel Clinical Research
- CenterWatch
- ClinChoice
- Elligo Health Research
- FOMAT Medical Research
- Grand View Research
- ICON plc
- InsightAce Analytic
- IQVIA
- KV Clinical Research
- Parexel
- Precision Site Network
- Premier Research
- SGS Société Générale de Surveillance
- SMO-Pharmina
- Sofpromed
- The Aurum Institute
- Velocity Clinical Research
- WCG Clinical
- Xylem Research
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- CenExel Clinical Research
- CenterWatch
- ClinChoice
- Elligo Health Research
- FOMAT Medical Research
- Grand View Research
- ICON plc
- InsightAce Analytic
- IQVIA
- KV Clinical Research
- Parexel
- Precision Site Network
- Premier Research
- SGS Société Générale de Surveillance
- SMO-Pharmina
- Sofpromed
- The Aurum Institute
- Velocity Clinical Research
- WCG Clinical
- Xylem Research
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 381 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
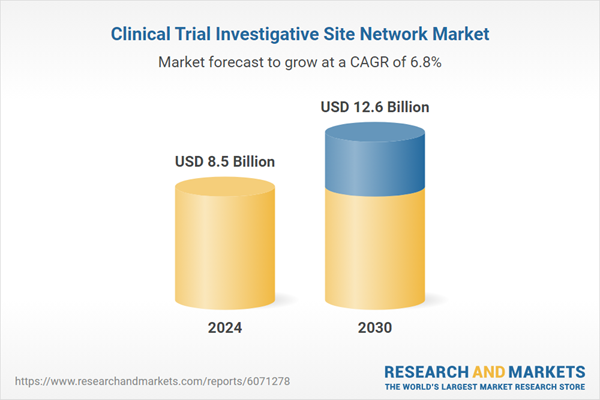
| Estimated Market Value ( USD | $ 8.5 Billion |
| Forecasted Market Value ( USD | $ 12.6 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |