Global Pediatric Catheters Market - Key Trends & Drivers Summarized
Why Are Pediatric Catheters Emerging as a Critical Cornerstone in Specialized Childcare?
Pediatric catheters have become essential tools in the clinical management of infants and children across a broad range of medical conditions, from acute emergencies to long-term chronic care. Unlike adult catheterization procedures, pediatric applications demand highly specialized products that are meticulously engineered for smaller anatomies, fragile vasculature, and developmentally specific physiological responses. Catheters are used for a wide array of pediatric interventions including intravenous therapy, hemodialysis, peritoneal dialysis, urinary drainage, enteral feeding, and central venous access. These devices are particularly vital in neonatal intensive care units (NICUs), pediatric intensive care units (PICUs), and pediatric oncology wards, where they facilitate critical therapies such as chemotherapy, parenteral nutrition, and long-term antimicrobial infusions. The clinical precision required for pediatric catheter insertion, maintenance, and removal has driven the development of highly flexible, biocompatible, and miniaturized catheter variants with pressure-regulated valves, anti-kink designs, and integrated securement features. Innovations in catheter tip design and insertion techniques are helping reduce complications such as phlebitis, infiltration, occlusion, and catheter-associated infections. Moreover, the clinical emphasis on reducing needle sticks and procedural stress among children has elevated the role of catheters in delivering pain-free, continuous access to medication and fluids. Pediatric care teams now rely on a wide array of catheter types - ranging from peripherally inserted central catheters (PICCs) and tunneled central lines to umbilical catheters and Foley urinary catheters - all tailored to meet age-specific needs. As pediatric medicine becomes more specialized, the demand for child-specific catheter technologies continues to rise, pushing innovation and adoption across hospitals and outpatient care settings worldwide.How Is Product Engineering Revolutionizing the Safety and Usability of Pediatric Catheters?
The evolution of pediatric catheter design is being driven by rapid advancements in biomaterials, ergonomics, and infection control strategies. Historically, pediatric catheterization faced multiple challenges, including risk of vessel trauma, catheter displacement, and high infection rates due to prolonged use. Today's catheters are manufactured using medical-grade silicone, polyurethane, and other biocompatible polymers that offer a delicate balance between flexibility, durability, and chemical resistance. Innovations such as pressure-activated safety valves, antimicrobial coatings, and thermosensitive materials are significantly reducing complications and enhancing patient comfort. Some modern catheters now include heparin-bonded surfaces or silver-impregnated components designed to inhibit microbial colonization and thrombus formation, which is particularly critical in pediatric oncology and NICU environments. Technological integration is also being seen in the form of embedded RFID chips and color-coded lumens that assist caregivers in managing multi-lumen catheter systems more efficiently. Manufacturers are increasingly adopting atraumatic insertion tips, low-profile ports, and soft hub connectors to improve securement and reduce accidental dislodgement during routine child movement. Catheter systems are now also optimized for use with ultrasound-guided insertion and securement devices, reducing dependence on anatomical landmarks and minimizing the need for repeat attempts. Furthermore, catheter kits customized for pediatric use - including size-specific insertion tools, dressing packs, and flushing accessories - are becoming standard offerings from leading medical device companies. These engineering advancements are playing a transformative role in addressing the unique anatomical and physiological needs of children, while also simplifying workflows for healthcare professionals managing high-risk pediatric populations.Why Are Hospitals, Homecare, and Ambulatory Settings Creating New Demand Dimensions?
The use of pediatric catheters is no longer confined to high-acuity hospital environments. With the shift toward decentralized healthcare delivery, the demand for catheter-based interventions in ambulatory care centers, homecare programs, and palliative settings has surged. This expansion has placed a renewed focus on product portability, ease of maintenance, and patient-friendly features, particularly for chronic disease management in children with conditions like cystic fibrosis, spina bifida, end-stage renal disease, and gastrointestinal anomalies. For home-based patients requiring dialysis, enteral feeding, or medication infusions, pediatric catheter systems must be designed to minimize infection risk while allowing caregivers and parents to manage them with minimal clinical supervision. Catheter education programs, remote monitoring technologies, and mobile apps for access tracking are being integrated into home healthcare protocols to support compliance and reduce hospital readmissions. In ambulatory surgery centers and outpatient oncology clinics, the use of temporary and mid-term catheters has increased as more pediatric procedures are performed in day-care settings to minimize hospitalization. Schools and rehabilitation centers are also seeking child-safe and discreet catheter solutions that do not interfere with mobility or learning. Pediatric urology is another area witnessing an uptick in intermittent catheter use, supported by newer, hydrophilic-coated catheters that require no manual lubrication and offer smooth, pain-free insertion. In all these care environments, ease of training, infection prevention, and product reliability are paramount. These end-use dynamics are prompting catheter manufacturers to diversify their product portfolios to serve an increasingly fragmented yet specialized pediatric care continuum spanning hospital, clinic, and home environments.The Growth in the Pediatric Catheters Market Is Driven by Several Factors…
The growth in the pediatric catheters market is driven by several factors anchored in product innovation, expanded clinical applications, and evolving treatment models. A major driver is the increasing prevalence of pediatric chronic conditions and congenital anomalies that require prolonged or repetitive access to the vascular, urinary, or gastrointestinal systems - thus fueling demand for safe, long-dwell catheter solutions. Technological advancements in catheter materials, such as anti-infective coatings, kink-resistant shafts, and thermoplastic polymers, are enabling longer indwelling times with lower complication rates, making these devices suitable for both acute and long-term pediatric care. From an end-use standpoint, the rapid expansion of NICUs, PICUs, and pediatric specialty units in emerging and developed markets is creating consistent demand for a wide range of catheter types tailored to neonates, infants, and adolescents. Moreover, the rise of outpatient care models, including pediatric day-care surgeries and home-based infusion therapy programs, is expanding usage beyond traditional hospital environments and requiring more user-friendly, portable, and durable catheter systems. On the consumer behavior front, rising parental involvement in care decisions is encouraging manufacturers to design products that are less intimidating, easier to handle, and child-centric in both function and form. Training tools, educational apps, and video guides are being used to empower parents and caregivers, particularly for home management of central venous or enteral access. Regulatory shifts and reimbursement reforms across regions are also improving access to advanced catheter systems by supporting procurement in public hospitals and homecare programs. In addition, growing collaborations between pediatric device startups and academic institutions are accelerating the pipeline of next-generation catheter solutions designed specifically for younger patients. Collectively, these factors are driving sustained and diversified growth in the pediatric catheters market, reinforcing its pivotal role in modern pediatric medicine.Report Scope
The report analyzes the Pediatric Catheters market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Cardiovascular Catheters, Urology Catheters, Intravenous Catheters, Neurovascular Catheters, Specialty Catheters).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Cardiovascular Catheters segment, which is expected to reach US$4.3 Billion by 2030 with a CAGR of a 6%. The Urology Catheters segment is also set to grow at 9.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.3 Billion in 2024, and China, forecasted to grow at an impressive 11.2% CAGR to reach $2.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pediatric Catheters Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pediatric Catheters Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pediatric Catheters Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 180 Medical, Inc., Abbott Laboratories, B. Braun Melsungen AG, Becton, Dickinson and Company (BD), Boston Scientific Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Pediatric Catheters market report include:
- 180 Medical, Inc.
- Abbott Laboratories
- B. Braun Melsungen AG
- Becton, Dickinson and Company (BD)
- Boston Scientific Corporation
- C. R. Bard (now part of BD)
- Cardinal Health
- Coloplast Corp
- Convatec Group
- Cook Medical
- Cure Medical
- Edwards Lifesciences Corporation
- Getinge AB
- Hollister Incorporated
- Johnson & Johnson Services, Inc.
- Koninklijke Philips N.V.
- Medline Industries, LP
- Medtronic plc
- Smiths Medical
- Teleflex Incorporated
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 180 Medical, Inc.
- Abbott Laboratories
- B. Braun Melsungen AG
- Becton, Dickinson and Company (BD)
- Boston Scientific Corporation
- C. R. Bard (now part of BD)
- Cardinal Health
- Coloplast Corp
- Convatec Group
- Cook Medical
- Cure Medical
- Edwards Lifesciences Corporation
- Getinge AB
- Hollister Incorporated
- Johnson & Johnson Services, Inc.
- Koninklijke Philips N.V.
- Medline Industries, LP
- Medtronic plc
- Smiths Medical
- Teleflex Incorporated
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 195 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
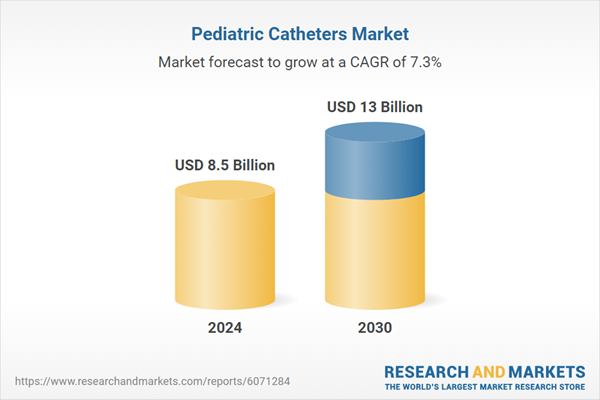
| Estimated Market Value ( USD | $ 8.5 Billion |
| Forecasted Market Value ( USD | $ 13 Billion |
| Compound Annual Growth Rate | 7.3% |
| Regions Covered | Global |