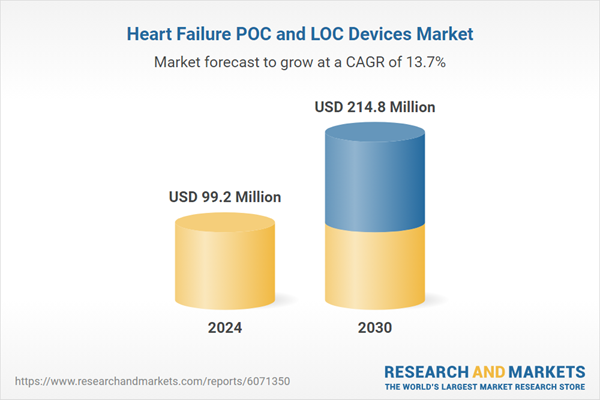
Global Heart Failure POC and LOC Devices Market - Key Trends & Drivers Summarized
Why Are Point-of-Care and Lab-on-a-Chip Devices Critical in Heart Failure Management?
Heart failure remains a leading cause of morbidity and mortality worldwide, creating a growing need for rapid diagnostic and monitoring solutions. Point-of-care (POC) and lab-on-a-chip (LOC) devices have emerged as crucial technologies in heart failure management, offering real-time, decentralized testing and reducing reliance on traditional laboratory diagnostics. These devices enable early detection of biomarkers such as B-type natriuretic peptide (BNP) and troponin, allowing physicians to assess cardiac function and disease progression more efficiently. The ability to conduct immediate, on-site tests in emergency settings, clinics, or even at home has significantly improved response times, leading to earlier interventions and better patient outcomes. Moreover, the increasing prevalence of chronic heart disease, lifestyle-related risk factors, and aging populations has heightened the demand for accessible, portable, and cost-effective cardiac monitoring tools. As the healthcare industry shifts toward preventive care and personalized medicine, POC and LOC devices are playing a transformative role in heart failure diagnostics, making real-time patient monitoring more feasible and effective.How Are Microfluidics, AI, and IoT Enhancing POC and LOC Devices for Heart Failure?
The integration of microfluidics, artificial intelligence (AI), and the Internet of Things (IoT) is revolutionizing heart failure POC and LOC device capabilities. Microfluidic lab-on-a-chip technology enables precise sample analysis using minimal blood or saliva, reducing the need for invasive procedures. AI-driven diagnostic algorithms enhance the accuracy of biomarker detection by analyzing patterns in test results and correlating them with patient history. IoT-enabled wearable biosensors, capable of continuously monitoring heart rate, blood pressure, and fluid retention, are providing real-time insights into a patient’ s cardiovascular health. These connected devices allow healthcare providers to remotely track patients and receive alerts for any abnormalities, enabling proactive interventions. Cloud-based data integration ensures seamless transmission of diagnostic information across healthcare systems, improving coordinated care and decision-making. With the growing emphasis on remote monitoring and telehealth, these technological advancements are making heart failure management more predictive and personalized, reducing hospital readmissions and improving overall survival rates.What Barriers Exist in the Adoption of POC and LOC Devices for Heart Failure?
While heart failure POC and LOC devices offer numerous advantages, several barriers hinder their widespread adoption. One of the primary challenges is regulatory approval, as cardiac diagnostics require stringent validation to ensure accuracy, reliability, and safety. The process of obtaining regulatory clearance from agencies like the FDA and EMA can be time-consuming, delaying the market entry of innovative devices. Another challenge is the cost of implementation, as advanced microfluidic and AI-powered diagnostic tools require significant investment in research, development, and manufacturing. Additionally, ensuring interoperability with existing electronic health records (EHRs) and healthcare systems remains a hurdle, as seamless data integration is crucial for real-time patient monitoring. There is also a need for greater clinician and patient awareness regarding the benefits of POC and LOC devices, as traditional diagnostic methods continue to dominate the market. Overcoming these challenges will require collaborative efforts between medical device manufacturers, regulatory bodies, and healthcare providers to ensure broader accessibility and adoption.What Is Driving the Growth of the Heart Failure POC and LOC Devices Market?
The growth in the heart failure POC and LOC devices market is driven by the increasing burden of cardiovascular diseases, advancements in microfluidics and biosensor technology, and the expansion of telehealth services. Rising healthcare expenditures and the shift toward early detection and preventive care have fueled demand for rapid and decentralized diagnostic solutions. The growing focus on home-based monitoring and remote patient management has accelerated the adoption of portable and wearable heart failure monitoring devices. Government initiatives aimed at reducing cardiovascular-related hospitalizations and healthcare costs are also playing a significant role in driving market expansion. As technology continues to advance, the heart failure POC and LOC devices market is set to grow, revolutionizing how cardiovascular diseases are diagnosed and managed worldwide.Report Scope
The report analyzes the Heart Failure POC and LOC Devices market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Test Type (Proteomic Testing Type, Metabolomic Testing Type, Genomic Testing Type); Technology (Microfluidics Technology, Array-based Systems Technology, Other Technologies); End-Use (Clinics End-Use, Hospitals End-Use, Home End-Use, Assisted Living Healthcare Facilities End-Use, Laboratory End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Proteomic Testing segment, which is expected to reach US$133.6 Million by 2030 with a CAGR of a 14.3%. The Metabolomic Testing segment is also set to grow at 12.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $27.0 Million in 2024, and China, forecasted to grow at an impressive 18.4% CAGR to reach $46.0 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Heart Failure POC and LOC Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Heart Failure POC and LOC Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Heart Failure POC and LOC Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Akouos, Amplifon S.p.A., AudioCure Pharma, Cilcare, Cochlear Limited and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Heart Failure POC and LOC Devices market report include:
- Abbott Laboratories
- ACON Laboratories, Inc.
- Alfa Scientific Designs, Inc.
- American Screening Corporation
- Baxter International Inc.
- Beckman Coulter, Inc.
- Becton, Dickinson and Company
- Bio-Rad Laboratories
- CardioSignal
- Danaher Corporation
- Diagnoptics Technologies BV
- F. Hoffmann-La Roche Ltd
- Koninklijke Philips N.V.
- Lepu Medical Technology
- Medtronic plc
- Murata Vios, Inc.
- Nano-Ditech Corporation
- Nova Biomedical
- Siemens Healthineers
- Ventric Health
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- ACON Laboratories, Inc.
- Alfa Scientific Designs, Inc.
- American Screening Corporation
- Baxter International Inc.
- Beckman Coulter, Inc.
- Becton, Dickinson and Company
- Bio-Rad Laboratories
- CardioSignal
- Danaher Corporation
- Diagnoptics Technologies BV
- F. Hoffmann-La Roche Ltd
- Koninklijke Philips N.V.
- Lepu Medical Technology
- Medtronic plc
- Murata Vios, Inc.
- Nano-Ditech Corporation
- Nova Biomedical
- Siemens Healthineers
- Ventric Health
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 387 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 99.2 Million |
| Forecasted Market Value ( USD | $ 214.8 Million |
| Compound Annual Growth Rate | 13.7% |
| Regions Covered | Global |