Global Respiratory Syncytial Virus Diagnostics Market - Key Trends & Drivers Summarized
Why Is Early and Accurate Diagnosis of Respiratory Syncytial Virus (RSV) More Critical Than Ever?
Respiratory Syncytial Virus (RSV) is a highly contagious virus that causes severe respiratory infections, particularly in infants, the elderly, and immunocompromised individuals. As RSV remains a leading cause of hospitalizations in young children and contributes to significant morbidity in older adults, the demand for rapid and accurate diagnostics has surged. The seasonal nature of RSV infections, coupled with its ability to mimic other respiratory illnesses such as influenza and COVID-19, underscores the need for advanced diagnostic tools that can differentiate RSV from other viral pathogens. Moreover, the recent emergence of RSV outbreaks and co-infections with other respiratory viruses has further increased the urgency for reliable diagnostic solutions. Governments, healthcare institutions, and diagnostic companies are investing heavily in RSV detection technologies to facilitate early intervention, reduce transmission rates, and improve patient outcomes.How Are Innovations in RSV Diagnostic Technologies Enhancing Detection and Accuracy?
The landscape of RSV diagnostics has evolved significantly with the advent of molecular testing, point-of-care diagnostics, and AI-powered diagnostic platforms. Traditional methods such as viral culture and direct fluorescent antibody (DFA) testing are being phased out in favor of more sensitive and rapid molecular assays. Polymerase chain reaction (PCR)-based diagnostics have emerged as the gold standard for RSV detection, offering high accuracy and the ability to detect even low viral loads. Additionally, the development of isothermal amplification techniques, such as loop-mediated isothermal amplification (LAMP), has facilitated rapid and cost-effective RSV diagnosis, particularly in resource-limited settings. The integration of AI and machine learning in diagnostic imaging and digital pathology is further improving test interpretation, enhancing diagnostic precision, and minimizing false negatives. As RSV testing capabilities advance, healthcare providers are better equipped to detect infections early and implement appropriate treatment strategies.What Market Trends Are Driving the Demand for RSV Diagnostics?
The growing awareness of RSV-related complications and the increasing adoption of point-of-care testing (POCT) have significantly contributed to market expansion. The COVID-19 pandemic has further accelerated interest in multiplex respiratory virus panels, which simultaneously test for RSV, influenza, and SARS-CoV-2, enabling comprehensive respiratory disease management. Additionally, the rising prevalence of RSV infections in aging populations has increased the need for routine RSV screening in long-term care facilities and hospitals. The expansion of home-based and decentralized testing solutions has also gained traction, with self-testing kits and mobile diagnostic platforms allowing for convenient RSV detection outside traditional healthcare settings. Furthermore, government initiatives promoting RSV surveillance programs and the integration of RSV diagnostics into national immunization strategies are expected to boost market growth. As consumer demand for fast and reliable respiratory virus testing rises, diagnostic manufacturers are continuously innovating to improve test accessibility and performance.What Are the Key Growth Drivers of the Respiratory Syncytial Virus Diagnostics Market?
The growth in the global respiratory syncytial virus diagnostics market is driven by several factors, including the increasing incidence of RSV infections, advancements in molecular diagnostics, and the rising demand for point-of-care and home-based testing solutions. The expansion of multiplex testing panels, which enable simultaneous detection of multiple respiratory pathogens, has further fueled market demand. Additionally, the growing adoption of AI-driven diagnostic tools and digital pathology platforms is enhancing test accuracy and efficiency. The rising investment in RSV surveillance programs and government-led initiatives to improve disease monitoring are also contributing to market expansion. Furthermore, the ongoing development of RSV vaccines and antiviral treatments is expected to drive demand for complementary diagnostic solutions. As the healthcare industry continues to prioritize infectious disease management, the RSV diagnostics market is poised for sustained growth, offering innovative solutions for early detection, disease prevention, and improved patient outcomes.Report Scope
The report analyzes the Respiratory Syncytial Virus Diagnostics market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Direct Fluorescent Antibody Method, Rapid Antigen Diagnostic Test, Molecular Diagnostics, Chromatographic Immunoassay, Diagnostic Imaging, Gel Microdroplets, Flow Cytometry, Others); Vertical (Hospitals, Laboratory, Clinics, Homecare).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Some of the 37 companies featured in this Respiratory Syncytial Virus Diagnostics market report include -
- Abbott Laboratories
- Affymetrix (part of Thermo Fisher)
- Alere Inc.
- Becton, Dickinson and Company (BD)
- Biocartis NV
- BioMérieux Inc.
- bioMérieux SA
- Bio-Rad Laboratories Inc.
- Cepheid
- Coris BioConcept
- Danaher Corporation
- DiaSorin S.p.A.
- F. Hoffmann-La Roche Ltd
- Fast-track Diagnostics
- GenMark Diagnostics
- Grifols S.A.
- Hologic Inc.
- Luminex Corporation
- Meridian Bioscience, Inc.
- MilliporeSigma
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Direct Fluorescent Antibody Method segment, which is expected to reach US$1.9 Billion by 2030 with a CAGR of a 3.1%. The Rapid Antigen Diagnostic Test segment is also set to grow at 3.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.7 Billion in 2024, and China, forecasted to grow at an impressive 7.2% CAGR to reach $1.5 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Respiratory Syncytial Virus Diagnostics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Respiratory Syncytial Virus Diagnostics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Respiratory Syncytial Virus Diagnostics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Air Liquide S.A., Becton, Dickinson and Company, Besmed Health Business Corp., CAIRE Inc., Drägerwerk AG & Co. KGaA and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 37 Featured):
- Abbott Laboratories
- Affymetrix (part of Thermo Fisher)
- Alere Inc.
- Becton, Dickinson and Company (BD)
- Biocartis NV
- BioMérieux Inc.
- bioMérieux SA
- Bio-Rad Laboratories Inc.
- Cepheid
- Coris BioConcept
- Danaher Corporation
- DiaSorin S.p.A.
- F. Hoffmann-La Roche Ltd
- Fast-track Diagnostics
- GenMark Diagnostics
- Grifols S.A.
- Hologic Inc.
- Luminex Corporation
- Meridian Bioscience, Inc.
- MilliporeSigma
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Affymetrix (part of Thermo Fisher)
- Alere Inc.
- Becton, Dickinson and Company (BD)
- Biocartis NV
- BioMérieux Inc.
- bioMérieux SA
- Bio-Rad Laboratories Inc.
- Cepheid
- Coris BioConcept
- Danaher Corporation
- DiaSorin S.p.A.
- F. Hoffmann-La Roche Ltd
- Fast-track Diagnostics
- GenMark Diagnostics
- Grifols S.A.
- Hologic Inc.
- Luminex Corporation
- Meridian Bioscience, Inc.
- MilliporeSigma
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 292 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
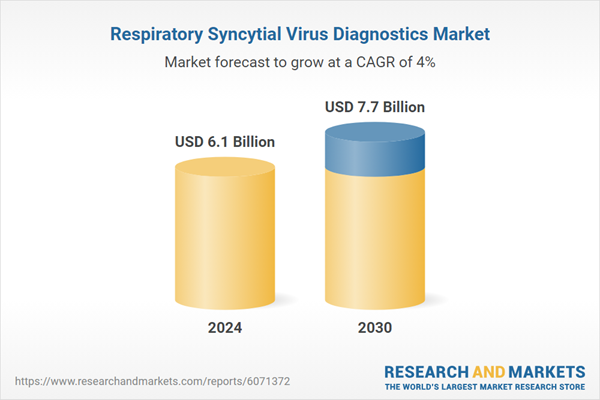
| Estimated Market Value ( USD | $ 6.1 Billion |
| Forecasted Market Value ( USD | $ 7.7 Billion |
| Compound Annual Growth Rate | 4.0% |
| Regions Covered | Global |