Global Sickle Cell Anemia Testing and Screening Market - Key Trends & Drivers Summarized
Why Is Sickle Cell Anemia Testing Becoming a Global Healthcare Priority? Understanding the Rising Demand for Early Diagnosis
Sickle cell anemia testing and screening have gained increasing importance as governments and healthcare institutions prioritize early detection and disease management. Sickle cell disease (SCD) is a genetic blood disorder that affects millions worldwide, particularly in regions with high prevalence rates, such as Africa, the Middle East, India, and parts of North and South America. Early diagnosis is crucial in preventing complications such as stroke, organ damage, and severe pain crises. Governments and health organizations have ramped up newborn screening programs to ensure early intervention, leading to improved patient outcomes and reduced healthcare costs. Additionally, the expansion of premarital and prenatal screening initiatives has further reinforced the need for accessible and accurate testing solutions. As awareness of sickle cell disease grows, healthcare systems are integrating advanced screening technologies to facilitate early identification and better treatment planning.How Are Technological Advancements Improving Sickle Cell Testing? Exploring Innovations in Diagnostic Accuracy and Accessibility
Advancements in diagnostic technology have revolutionized sickle cell anemia testing, enabling more precise, rapid, and cost-effective screening methods. Traditional laboratory tests, such as hemoglobin electrophoresis and high-performance liquid chromatography (HPLC), have been complemented by next-generation diagnostic tools, including point-of-care (POC) testing devices, automated molecular assays, and genetic screening techniques. CRISPR-based gene-editing research has also opened possibilities for identifying genetic mutations associated with sickle cell disease, paving the way for precision medicine approaches. Furthermore, AI-powered diagnostic platforms are enhancing interpretation accuracy, reducing false positives and negatives. The expansion of decentralized testing options, including at-home and community-based screening kits, has made testing more accessible in remote areas with limited healthcare infrastructure. As testing technology continues to evolve, sickle cell anemia diagnostics are becoming more efficient, affordable, and globally accessible.What Challenges Are Hindering the Adoption of Sickle Cell Testing? Addressing Market Barriers and Healthcare Disparities
Despite the advancements in testing technology, several challenges limit the widespread adoption of sickle cell anemia screening. Limited healthcare infrastructure in low-income regions has hindered the availability of diagnostic tools, restricting access to timely detection and treatment. The high costs associated with molecular and genetic screening methods also pose affordability concerns, making it difficult for underprivileged populations to access quality care. Additionally, inadequate awareness about sickle cell disease and testing options has led to underdiagnosis and delayed interventions, particularly in developing nations. Regulatory hurdles and inconsistent testing guidelines across different regions further complicate market expansion, requiring greater international collaboration to standardize screening programs. Addressing these challenges will require targeted investments in healthcare infrastructure, policy reforms, and public health campaigns to increase accessibility and awareness.What's Driving the Growth of the Sickle Cell Anemia Testing Market? Identifying Key Expansion Trends and Healthcare Initiatives
The growth in the sickle cell anemia testing and screening market is driven by several factors, including government-backed screening programs, advancements in molecular diagnostics, and increased healthcare funding. National newborn screening mandates have played a crucial role in expanding early detection efforts, prompting investments in advanced diagnostic technologies. The rising prevalence of sickle cell disease in developing countries has further accelerated the demand for affordable and scalable screening solutions. Additionally, global health organizations and non-profit initiatives are funding research and community-based programs to enhance disease awareness and testing accessibility. The expansion of genetic testing services and CRISPR-based innovations has also contributed to market growth, as personalized medicine approaches gain traction in managing sickle cell disease. With ongoing advancements in diagnostics and increasing global efforts to improve disease management, the market for sickle cell anemia testing is expected to witness sustained growth, improving patient outcomes worldwide.Report Scope
The report analyzes the Sickle Cell Anemia Testing and Screening market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Technology (Hemoglobin, High-Performance Liquid Chromatography, Point-of-Care Tests, Other Tests); Age Group (Below 1 yr, 25 - 60 yrs, Others); Sector Type (Government Labs, Private Labs, Corporate Labs, PPP).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Some of the 32 companies featured in this Sickle Cell Anemia Testing and Screening market report include -
- Alpha Laboratories
- ASI (Analytical Services International)
- Atlas Medical
- Beam Therapeutics
- BioMedomics Inc.
- Bio-Rad Laboratories, Inc
- bluebird bio Inc.
- Bristol-Myers Squibb Company
- Cigna
- CRISPR Therapeutics
- Daktari Diagnostics
- Emmaus Life Sciences, Inc.
- Fisher Scientific
- Functional Fluidics
- Global Blood Therapeutics, Inc. (Pfizer Inc.)
- Hemex Health
- HEMEX HEALTH
- HemoTypeSC
- Imara Inc.
- KovaDx
- Laboratory Corporation of America Holdings
- LABS Inc.
- Mayo Clinic
- Modus Therapeutics
- Novartis AG
- Pfizer Inc.
- Quest Diagnostics
- Sanofi SA
- Silver Lake Research Corporation
- STRECK, INC
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Hemoglobin Technology segment, which is expected to reach US$338.3 Million by 2030 with a CAGR of a 9.2%. The High-Performance Liquid Chromatography Technology segment is also set to grow at 8.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $112.5 Million in 2024, and China, forecasted to grow at an impressive 13% CAGR to reach $144.8 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Sickle Cell Anemia Testing and Screening Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Sickle Cell Anemia Testing and Screening Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Sickle Cell Anemia Testing and Screening Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Bigo Live, Byte, Chingari, Clapper, Dubsmash and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 32 Featured):
- Alpha Laboratories
- ASI (Analytical Services International)
- Atlas Medical
- Beam Therapeutics
- BioMedomics Inc.
- Bio-Rad Laboratories, Inc
- bluebird bio Inc.
- Bristol-Myers Squibb Company
- Cigna
- CRISPR Therapeutics
- Daktari Diagnostics
- Emmaus Life Sciences, Inc.
- Fisher Scientific
- Functional Fluidics
- Global Blood Therapeutics, Inc. (Pfizer Inc.)
- Hemex Health
- HEMEX HEALTH
- HemoTypeSC
- Imara Inc.
- KovaDx
- Laboratory Corporation of America Holdings
- LABS Inc.
- Mayo Clinic
- Modus Therapeutics
- Novartis AG
- Pfizer Inc.
- Quest Diagnostics
- Sanofi SA
- Silver Lake Research Corporation
- STRECK, INC
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alpha Laboratories
- ASI (Analytical Services International)
- Atlas Medical
- Beam Therapeutics
- BioMedomics Inc.
- Bio-Rad Laboratories, Inc
- bluebird bio Inc.
- Bristol-Myers Squibb Company
- Cigna
- CRISPR Therapeutics
- Daktari Diagnostics
- Emmaus Life Sciences, Inc.
- Fisher Scientific
- Functional Fluidics
- Global Blood Therapeutics, Inc. (Pfizer Inc.)
- Hemex Health
- HEMEX HEALTH
- HemoTypeSC
- Imara Inc.
- KovaDx
- Laboratory Corporation of America Holdings
- LABS Inc.
- Mayo Clinic
- Modus Therapeutics
- Novartis AG
- Pfizer Inc.
- Quest Diagnostics
- Sanofi SA
- Silver Lake Research Corporation
- STRECK, INC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 371 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
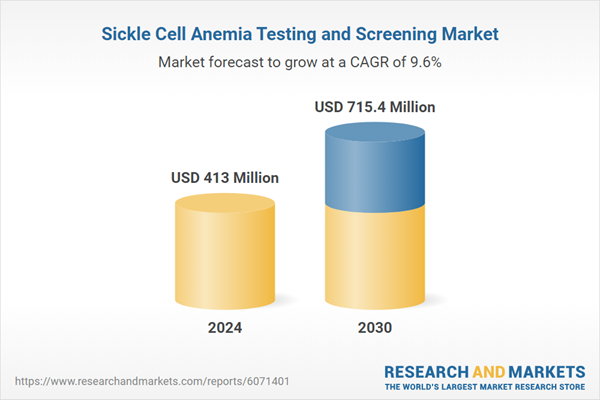
| Estimated Market Value ( USD | $ 413 Million |
| Forecasted Market Value ( USD | $ 715.4 Million |
| Compound Annual Growth Rate | 9.6% |
| Regions Covered | Global |