Global Urinary Tract Infection (UTI) Testing Market - Key Trends & Drivers Summarized
Is the Rising Global Burden of UTIs Fueling a Surge in Diagnostic Demand?
Urinary tract infections (UTIs) are among the most common bacterial infections globally, impacting hundreds of millions of people annually and leading to a substantial increase in diagnostic testing. The growing incidence of UTIs across all age groups - particularly among women, the elderly, and individuals with diabetes, kidney conditions, or immunocompromised states - is driving sustained demand for rapid and accurate testing solutions. As awareness of early diagnosis and antimicrobial stewardship increases, healthcare systems are investing more in point-of-care diagnostics to reduce the burden on hospitals and minimize the overprescription of antibiotics. Recurrent and complicated UTIs, often associated with catheter use or structural abnormalities in the urinary tract, further contribute to the growing volume of diagnostic procedures in both inpatient and outpatient settings. Additionally, the global rise in healthcare-acquired infections (HAIs), particularly in long-term care facilities, is heightening the need for reliable UTI screening protocols. As clinicians aim to differentiate between asymptomatic bacteriuria and true infection, demand is shifting toward more specific and sensitive tests that can accurately guide treatment decisions. This evolving clinical landscape is pushing diagnostic laboratories, primary care providers, and urgent care centers to expand their capabilities in UTI detection, driving growth in the global UTI testing market.How Are Technological Advancements and POC Diagnostics Transforming UTI Testing?
Technological innovation is dramatically reshaping the urinary tract infection testing landscape, with a growing emphasis on speed, accuracy, and accessibility. Traditional culture-based methods, though highly reliable, require 24-48 hours for results and are gradually being supplemented or replaced by rapid diagnostics. Modern urine dipstick tests and urinalysis kits now offer quicker detection of nitrites, leukocyte esterase, and other key indicators of infection, making them valuable tools in emergency departments and primary care settings. Additionally, molecular diagnostics - particularly PCR-based tests - are enabling the rapid identification of specific pathogens and resistance genes, significantly improving the precision of diagnosis and supporting appropriate antibiotic prescribing. Point-of-care (POC) platforms are becoming increasingly popular due to their portability, user-friendliness, and ability to deliver real-time results, particularly in rural or resource-limited environments. Automated urine analyzers are also being integrated into clinical laboratories to streamline workflows, reduce manual error, and allow for high-throughput testing. Furthermore, smartphone-connected devices and AI-driven diagnostic tools are emerging as novel solutions for home-based UTI monitoring, especially for patients with chronic or recurrent infections. These technological advancements are not only optimizing diagnostic accuracy but also helping clinicians move toward evidence-based, individualized treatment strategies, ultimately reducing the burden of antibiotic resistance and recurrent infections.Are Shifting Healthcare Models and Consumer Behaviors Expanding the Market for UTI Testing?
As healthcare systems evolve toward decentralized, patient-centered care, urinary tract infection testing is increasingly being conducted in non-traditional settings such as retail clinics, home testing environments, and telehealth-driven diagnostic platforms. Consumers today demand convenience, speed, and confidentiality - especially for sensitive conditions like UTIs. This has led to the proliferation of at-home UTI test kits, which can be purchased over the counter or online, offering privacy and rapid self-screening. These kits often utilize colorimetric strip technologies or app-connected sensors that help users interpret results accurately and decide when to seek medical attention. Telemedicine providers have also embraced UTI testing as a high-demand service, often prescribing antibiotics based on symptoms and test confirmations without the need for an in-person visit. Additionally, employers and universities are offering UTI screening services as part of occupational health and wellness programs, further expanding testing beyond traditional clinical environments. On the institutional side, the emphasis on early detection and infection control in hospitals, nursing homes, and rehabilitation centers is driving routine screening and post-catheterization monitoring. This expansion of care settings - alongside greater patient involvement in health management - is fostering a dynamic and rapidly diversifying market for UTI testing, encouraging the development of new test formats and distribution models to meet this demand.What Is Driving the Global Growth of the Urinary Tract Infection Testing Market?
The growth in the urinary tract infection testing market is driven by multiple converging factors tied to clinical need, technological progress, demographic trends, and healthcare delivery evolution. A major driver is the global increase in UTI prevalence, particularly among women, the elderly, and individuals with chronic illnesses or indwelling catheters, all of whom represent a consistent and expanding patient base. In parallel, rising awareness around the dangers of untreated UTIs - such as kidney infections, sepsis, and chronic recurrence - is prompting earlier and more frequent testing. The acceleration of antimicrobial resistance is also encouraging more precise diagnosis before antibiotic prescription, pushing healthcare providers to adopt rapid and pathogen-specific tests. The growing role of molecular and digital technologies, combined with the increased availability of affordable point-of-care and home-based test kits, is enhancing diagnostic reach and reducing access barriers. Favorable regulatory frameworks, supportive reimbursement policies, and public health initiatives promoting infectious disease surveillance are creating an enabling environment for testing innovation and market growth. Furthermore, the rise of telehealth, mobile diagnostics, and diagnostic-as-a-service (DaaS) platforms is expanding the availability of UTI testing to remote and underserved populations. As these trends continue to evolve, the urinary tract infection testing market is poised for strong and sustained expansion, driven by the combined imperatives of clinical urgency, patient empowerment, and diagnostic innovation.Report Scope
The report analyzes the Urinary Tract Infection Testing market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Urethritis, Cystitis, Pyelonephritis); End-Use (General Practitioners, Urologists, Urogynecologists, Hospital Laboratories, Reference Laboratories, Hospital Emergency Departments, Urgent Care, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Urethritis segment, which is expected to reach US$390 Million by 2030 with a CAGR of a 2.1%. The Cystitis segment is also set to grow at 3.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $166.9 Million in 2024, and China, forecasted to grow at an impressive 5.2% CAGR to reach $139.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Urinary Tract Infection Testing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Urinary Tract Infection Testing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Urinary Tract Infection Testing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AbbVie Inc., Astellas Pharma Inc., Axonics Modulation Technologies, Inc., B. Braun Melsungen AG, Becton, Dickinson and Company (BD) and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Urinary Tract Infection Testing market report include:
- Accelerate Diagnostics, Inc.
- Acutis Diagnostics
- Becton, Dickinson and Company (BD)
- Beckman Coulter
- bioMérieux
- Bio-Rad Laboratories, Inc.
- Danaher Corporation
- DiaSorin
- F. Hoffmann-La Roche Ltd.
- GENETWORx
- Healthy.io
- Patients Choice Laboratories
- QIAGEN
- Randox Laboratories Ltd.
- Siemens Healthineers
- T2 Biosystems, Inc.
- TestCard
- Thermo Fisher Scientific, Inc.
- Uritest Medical
- Winx
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Accelerate Diagnostics, Inc.
- Acutis Diagnostics
- Becton, Dickinson and Company (BD)
- Beckman Coulter
- bioMérieux
- Bio-Rad Laboratories, Inc.
- Danaher Corporation
- DiaSorin
- F. Hoffmann-La Roche Ltd.
- GENETWORx
- Healthy.io
- Patients Choice Laboratories
- QIAGEN
- Randox Laboratories Ltd.
- Siemens Healthineers
- T2 Biosystems, Inc.
- TestCard
- Thermo Fisher Scientific, Inc.
- Uritest Medical
- Winx
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 300 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
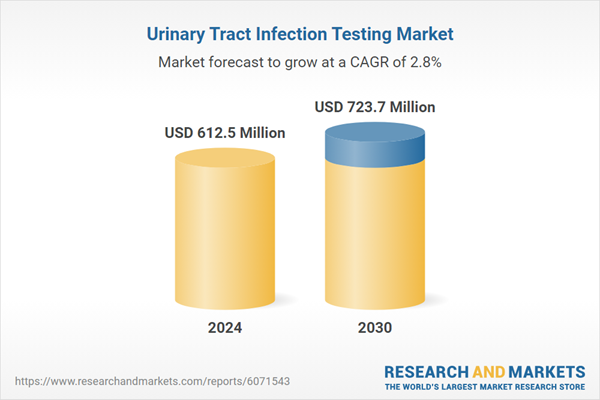
| Estimated Market Value ( USD | $ 612.5 Million |
| Forecasted Market Value ( USD | $ 723.7 Million |
| Compound Annual Growth Rate | 2.8% |
| Regions Covered | Global |