Global Vagus Nerve Stimulation Market - Key Trends & Drivers Summarized
Why Is Vagus Nerve Stimulation at the Forefront of Neuromodulation Breakthroughs?
Vagus nerve stimulation (VNS) has gained critical momentum in recent years as a transformative approach within the broader field of neuromodulation, offering hope in the treatment of complex neurological and psychiatric conditions. Originally approved for refractory epilepsy, VNS has since expanded its therapeutic reach to include treatment-resistant depression, migraine, cluster headaches, and even promising applications in inflammatory diseases and cardiac disorders. This growing clinical versatility stems from the vagus nerve's extensive physiological influence across the autonomic nervous system, enabling VNS to modulate brain circuits, neurotransmitter release, and inflammatory pathways. Modern VNS devices include both implantable and non-invasive systems, with transcutaneous VNS (tVNS) gaining favor for its ease of use and reduced procedural risks. These devices deliver controlled electrical impulses to the vagus nerve, either via surgical leads or external ear or neck-based electrodes. With an increasing number of patients seeking drug-free, long-term alternatives to conventional treatments, VNS has emerged as a viable solution, particularly in chronic and treatment-resistant conditions. As the body of clinical evidence grows, more physicians are integrating VNS into multidisciplinary care models, and patient interest is rising in parallel with expanded therapeutic indications. This shift underscores the growing importance of VNS as a pillar of personalized, neuro-targeted medicine.How Are Technological Innovations Enhancing the Efficacy and Accessibility of VNS Devices?
Technological advancements are playing a decisive role in redefining the capabilities and reach of vagus nerve stimulation devices. The latest generation of implantable VNS systems now feature responsive or closed-loop stimulation, allowing real-time adaptation of electrical pulses based on physiological markers such as heart rate or electroencephalographic data. These intelligent devices improve therapeutic outcomes while minimizing side effects, a crucial factor in patient adherence. Non-invasive options, particularly tVNS wearables, have introduced flexibility in treatment schedules, reducing the need for surgery and enabling at-home therapy. Battery life, device programmability, and miniaturization have also seen significant improvements, contributing to enhanced comfort and convenience for users. Integration with mobile applications and cloud-based analytics allows remote monitoring, clinician feedback, and individualized dosing regimens, opening doors for continuous care models. Some VNS platforms are exploring machine learning algorithms to fine-tune therapy parameters based on patient-specific responses. Additionally, device interoperability with other health technologies, such as digital mental health tools or sleep monitoring apps, is enabling more holistic patient support. These innovations are transforming VNS from a highly specialized hospital-based therapy into a broader, more adaptable solution with growing relevance in both outpatient and telehealth environments.What Role Do Regulatory Approvals and Research Pipelines Play in Market Maturation?
The regulatory and clinical trial landscape surrounding vagus nerve stimulation is rapidly evolving, fostering market expansion through increased trust, validation, and commercialization opportunities. The U.S. FDA and European CE Mark authorities have broadened approval for VNS devices across various indications, encouraging innovation among both established medtech companies and start-ups. For instance, non-invasive VNS devices have gained market entry for headache disorders and have received Breakthrough Device Designation for conditions such as PTSD and inflammatory bowel disease. These milestones not only validate efficacy and safety but also support accelerated pathways for further development. Concurrently, a robust pipeline of clinical trials is exploring VNS applications in disorders such as tinnitus, fibromyalgia, anxiety, rheumatoid arthritis, and even cognitive decline linked to Alzheimer's disease. This expanding portfolio of potential uses underscores the adaptability of VNS therapy and enhances its value proposition to investors, healthcare providers, and patients. Public and private funding for neuromodulation research is also growing, fueling academic-industry collaborations aimed at exploring next-gen VNS delivery mechanisms, including bioelectronic implants and gene-modulated neural interfaces. As these developments continue, the resulting data will solidify the role of VNS in clinical guidelines and encourage reimbursement frameworks to widen, further stimulating market adoption.What Factors Are Driving the Global Demand for Vagus Nerve Stimulation Solutions?
The growth in the vagus nerve stimulation market is driven by several factors directly linked to technological advancements, therapeutic diversification, changing treatment paradigms, and increasing patient engagement. The rising global incidence of neurological disorders such as epilepsy and depression - especially among aging and treatment-resistant populations - is creating sustained demand for alternative therapies that offer long-term relief without pharmacological burden. The expanding availability and acceptance of non-invasive VNS devices have lowered the entry barrier for patients and providers alike, fueling adoption across outpatient and home care settings. Increased awareness of bioelectronic medicine and the vagus nerve's regulatory role in inflammation, stress, and mood is also attracting new interest from both clinical and wellness perspectives. Healthcare systems and insurers are gradually aligning with neuromodulation as a cost-effective intervention, especially in cases where chronic medication and hospitalization costs are high. Furthermore, personalized medicine trends and the integration of digital health tools are empowering patients to manage their treatment more proactively, with VNS platforms increasingly offering real-time data and adaptive therapy. Rapid innovation from medtech companies, combined with favorable regulatory designations and growing investment in neurotechnology research, is expanding the market footprint across North America, Europe, and rapidly developing regions like Asia-Pacific. Collectively, these dynamics are positioning VNS as a powerful, scalable solution within the global therapeutic landscape.Report Scope
The report analyzes the Vagus Nerve Stimulation market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Implantable VNS Device, External VNS Device); Biomaterial (Metallic, Ceramics, Polymeric); Application (Epilepsy, Depression, Migraine); End-Use (Hospitals, Neurology Clinics, Ambulatory Surgery Centers, Research Centers).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Implantable VNS Device segment, which is expected to reach US$548.6 Million by 2030 with a CAGR of a 7.7%. The External VNS Device segment is also set to grow at 11.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $140.5 Million in 2024, and China, forecasted to grow at an impressive 12% CAGR to reach $171.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Vagus Nerve Stimulation Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Vagus Nerve Stimulation Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Vagus Nerve Stimulation Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alma Lasers, Almirall, Asclepion Laser Technologies, BTL Industries, Candela Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Vagus Nerve Stimulation market report include:
- BioControl Medical
- Boston Scientific Corporation
- Cirtec Medical
- Cyberonics, Inc.
- electroCore, Inc.
- Endonovo Therapeutics, Inc.
- EnteroMedics Inc.
- Helius Medical Technologies, Inc.
- Inspire Medical Systems, Inc.
- LivaNova PLC
- Masimo Corporation
- Medtronic plc
- MicroTransponder Inc.
- Nevro Corp.
- NeuroMetrix, Inc.
- NeuroPace, Inc.
- NeuroSigma, Inc.
- Parasym Ltd
- SetPoint Medical Corporation
- tVNS Health GmbH
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- BioControl Medical
- Boston Scientific Corporation
- Cirtec Medical
- Cyberonics, Inc.
- electroCore, Inc.
- Endonovo Therapeutics, Inc.
- EnteroMedics Inc.
- Helius Medical Technologies, Inc.
- Inspire Medical Systems, Inc.
- LivaNova PLC
- Masimo Corporation
- Medtronic plc
- MicroTransponder Inc.
- Nevro Corp.
- NeuroMetrix, Inc.
- NeuroPace, Inc.
- NeuroSigma, Inc.
- Parasym Ltd
- SetPoint Medical Corporation
- tVNS Health GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 476 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
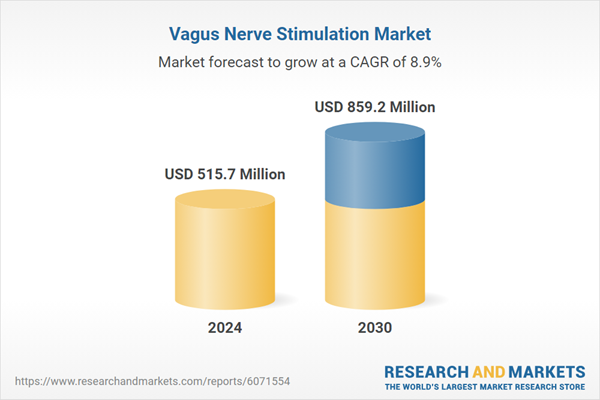
| Estimated Market Value ( USD | $ 515.7 Million |
| Forecasted Market Value ( USD | $ 859.2 Million |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |