Global Pediatric Diabetes Therapeutics Market - Key Trends & Drivers Summarized
Is the Pediatric Diabetes Landscape Shifting Toward More Personalized and Patient-Friendly Therapies?
Pediatric diabetes, particularly type 1 diabetes mellitus (T1DM), has witnessed a concerning rise globally, transforming what was once a niche therapeutic domain into a high-priority focus for health systems, pharmaceutical companies, and digital health innovators. Children and adolescents require disease management strategies that are fundamentally different from adults, due to fluctuating insulin requirements, developmental challenges, and behavioral patterns that complicate adherence. Over the past decade, significant strides have been made in developing more personalized and child-friendly diabetes therapeutics aimed at improving glycemic control while minimizing the burden of treatment. Insulin therapy continues to be the gold standard for pediatric T1DM, but innovation within this class has resulted in ultra-rapid acting insulins, long-acting basal analogs, and flexible dosing regimens that accommodate erratic eating and activity patterns typical of children. In parallel, combination therapies and off-label use of GLP-1 receptor agonists for type 2 diabetes in adolescents are gaining ground, particularly in the wake of rising obesity rates in this demographic. Importantly, there is growing focus on integrating behavioral health support, nutritional counseling, and patient education into therapeutic frameworks to ensure holistic disease management. As therapeutic delivery becomes more individualized and lifestyle-integrated, the traditional one-size-fits-all approach to pediatric diabetes is being replaced with precision-driven, age-tailored care models that prioritize quality of life alongside clinical outcomes.How Is Technology Redefining Therapeutic Delivery and Disease Monitoring in Pediatric Diabetes?
Technological innovation is playing a central role in transforming the pediatric diabetes therapeutics market, particularly by enabling real-time disease monitoring and seamless drug delivery mechanisms. The integration of continuous glucose monitoring (CGM) systems with insulin pumps has revolutionized diabetes management in children by reducing the need for frequent finger-pricks and allowing for dynamic insulin adjustments based on glucose trends. Hybrid closed-loop systems, often referred to as artificial pancreas technologies, are increasingly being adopted among pediatric patients, especially those with poor glycemic control or hypoglycemia unawareness. These systems combine CGM data with algorithms that automatically modulate insulin delivery, thereby minimizing hyperglycemic and hypoglycemic episodes. Wearable devices designed with child-specific ergonomics, wireless connectivity, and gamified interfaces are also contributing to higher engagement and better treatment adherence. Mobile apps and digital platforms now offer comprehensive diabetes management tools that include dose calculators, carbohydrate tracking, alerts for parents and caregivers, and telehealth support from endocrinologists. The availability of Bluetooth-enabled smart pens and app-connected meters is further enhancing real-time decision-making and treatment optimization. Additionally, remote monitoring has become especially critical for managing children in rural areas or during public health emergencies where in-person visits are restricted. All these innovations are allowing pediatric diabetes care to move beyond clinical environments into everyday settings, empowering both families and clinicians with actionable insights. As more devices become interoperable and artificial intelligence (AI) algorithms continue to mature, the pediatric therapeutics ecosystem is poised to become more predictive, automated, and proactive in managing this chronic condition.Why Are Therapeutic Approaches Expanding Beyond Insulin Alone?
While insulin remains the cornerstone of pediatric diabetes management, especially for T1DM, there is a growing push to diversify therapeutic strategies to include adjunctive and disease-modifying approaches. This trend is driven by recognition that insulin alone may not fully address the metabolic variability and autoimmune complexity seen in pediatric patients. In recent years, adjunctive therapies such as metformin, GLP-1 receptor agonists, and SGLT2 inhibitors have been explored in the pediatric population, particularly in type 2 diabetes and in insulin-resistant adolescents. Although regulatory approvals are still limited, ongoing clinical trials are evaluating the safety and efficacy of these agents for pediatric use, indicating a strong pipeline of future options. Immune-modulatory therapies aimed at preserving beta-cell function and delaying the onset of T1DM are another frontier under intense investigation, with several early-phase trials assessing monoclonal antibodies and antigen-specific immunotherapies in at-risk children. Nutraceuticals, microbiome-targeted therapies, and gene-based interventions are also emerging as potential areas of exploration in the long-term prevention and modulation of pediatric diabetes. Moreover, pharmaceutical companies are developing age-appropriate formulations - such as low-volume injectables, flavored oral suspensions, and long-acting delivery systems - that are easier for children to tolerate and manage. The evolving therapeutic landscape is not only addressing unmet medical needs but also aligning with the emotional, psychological, and developmental complexities of pediatric care. This shift reflects a deeper understanding that pediatric diabetes management must be multidimensional, extending beyond insulin replacement to embrace broader biological, behavioral, and environmental determinants of disease control.The Growth in the Pediatric Diabetes Therapeutics Market Is Driven by Several Factors…
The growth in the pediatric diabetes therapeutics market is driven by several factors centered around innovation in drug delivery, expansion of care settings, and rising demand for technology-integrated disease management. One of the key drivers is the increasing global incidence of both type 1 and type 2 diabetes among children, prompting healthcare systems to scale pediatric endocrinology services and invest in disease-specific treatment pathways. From a technological standpoint, the widespread adoption of continuous glucose monitors, insulin pumps, and closed-loop systems is fueling demand for compatible and algorithm-friendly insulin analogs, as well as AI-enabled therapy optimization tools. The rise of remote care models and telemedicine consultations is facilitating broader access to specialist care, particularly in underserved regions, and enabling sustained engagement through digital therapeutics. On the end-use front, pediatric hospitals, school-based health programs, and homecare setups are expanding their roles in therapy administration, monitoring, and compliance management. Consumer behavior trends also play a pivotal role, with families increasingly seeking flexible, user-friendly, and discreet therapeutic options that reduce stigma and disruption to children's daily activities. Pharmaceutical innovation is contributing to market growth through the development of ultra-rapid acting insulins, once-weekly injectables, and fixed-dose combinations designed specifically for adolescents. Regulatory support for pediatric-specific drug labels, fast-track approvals, and rare disease designations for early-onset diabetes subtypes are further accelerating the availability of targeted therapies. Additionally, collaborations between medtech firms, pharmaceutical companies, and patient advocacy groups are enhancing awareness, supporting trial participation, and enabling co-creation of patient-centric solutions. These converging trends are collectively driving strong momentum in the pediatric diabetes therapeutics market and setting the stage for continued innovation and expansion.Report Scope
The report analyzes the Pediatric Diabetes Therapeutics market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Type 1, Type 2); Application (Insulin, GLP-1 Receptor Agonists, Biguanide, SGLT2 Inhibitors, Others); Administration Route (Oral, Parenteral, Others); Distribution Channel (Hospital Pharmacies, Diagnostic Centers, Online Pharmacies).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Type 1 Diabetes segment, which is expected to reach US$11.3 Billion by 2030 with a CAGR of a 10.4%. The Type 2 Diabetes segment is also set to grow at 15.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.7 Billion in 2024, and China, forecasted to grow at an impressive 16.2% CAGR to reach $4.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pediatric Diabetes Therapeutics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pediatric Diabetes Therapeutics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pediatric Diabetes Therapeutics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abata Therapeutics, Amphastar Pharmaceuticals, Inc., AnTolRx, Inc., AstraZeneca, Bigfoot Biomedical and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Pediatric Diabetes Therapeutics market report include:
- Abata Therapeutics
- Amphastar Pharmaceuticals, Inc.
- AnTolRx, Inc.
- AstraZeneca
- Bigfoot Biomedical
- Biomea Fusion
- Boehringer Ingelheim International GmbH
- Capillary Biomedical
- Code Biotherapeutics
- Diasome Pharmaceuticals
- DiogenX
- Eli Lilly and Company
- Fractyl Health
- Glyscend Therapeutics
- Imcyse
- MannKind Corporation
- Novo Nordisk A/S
- Sanofi
- Vertex Pharmaceuticals Incorporated
- Xeris Pharmaceuticals
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abata Therapeutics
- Amphastar Pharmaceuticals, Inc.
- AnTolRx, Inc.
- AstraZeneca
- Bigfoot Biomedical
- Biomea Fusion
- Boehringer Ingelheim International GmbH
- Capillary Biomedical
- Code Biotherapeutics
- Diasome Pharmaceuticals
- DiogenX
- Eli Lilly and Company
- Fractyl Health
- Glyscend Therapeutics
- Imcyse
- MannKind Corporation
- Novo Nordisk A/S
- Sanofi
- Vertex Pharmaceuticals Incorporated
- Xeris Pharmaceuticals
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 464 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
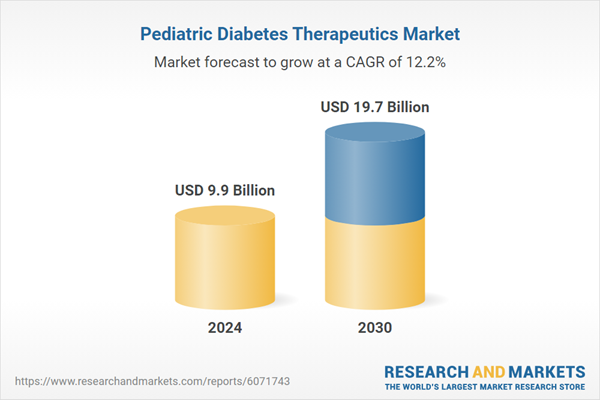
| Estimated Market Value ( USD | $ 9.9 Billion |
| Forecasted Market Value ( USD | $ 19.7 Billion |
| Compound Annual Growth Rate | 12.2% |
| Regions Covered | Global |