Global Recombinant Cell Culture Supplements Market - Key Trends & Drivers Summarized
Why Are Recombinant Cell Culture Supplements Revolutionizing Biopharmaceutical and Research Industries?
Recombinant cell culture supplements have become a cornerstone of modern biopharmaceutical production, regenerative medicine, and stem cell research, providing essential growth factors, hormones, and nutrients for cell culture systems. Unlike traditional serum-based supplements, recombinant cell culture supplements offer greater consistency, reduced risk of contamination, and improved scalability for large-scale manufacturing. The increasing demand for biologics, including monoclonal antibodies, vaccines, and gene therapies, has driven the adoption of recombinant supplements in bioprocessing. Additionally, advancements in tissue engineering and personalized medicine have further propelled the demand for high-quality, animal-free cell culture supplements that ensure reproducibility and regulatory compliance. As cell-based therapies and regenerative medicine gain traction, recombinant cell culture supplements are playing a crucial role in enabling innovative research and therapeutic development.How Are Technological Advancements Enhancing Recombinant Cell Culture Supplements?
Recent advancements in recombinant protein expression, purification, and formulation have significantly improved the efficacy and performance of cell culture supplements. The development of serum-free and chemically defined media has eliminated the variability associated with animal-derived components, ensuring more predictable and reproducible cell culture conditions. Innovations in protein engineering have also enabled the production of highly specific recombinant growth factors, cytokines, and hormones that optimize cell proliferation, differentiation, and productivity. Additionally, advancements in single-use bioprocessing and bioreactor technologies have facilitated large-scale production of recombinant supplements, improving cost-effectiveness and scalability. AI-driven predictive modeling and automation in cell culture optimization are further refining the formulation of recombinant supplements, accelerating research and commercial-scale biomanufacturing.What Market Trends Are Driving the Demand for Recombinant Cell Culture Supplements?
The increasing adoption of biologics, gene therapies, and cell-based assays in drug development has fueled demand for recombinant cell culture supplements. The shift toward animal-free and chemically defined culture media has been a key trend, driven by regulatory requirements for consistent, contamination-free biomanufacturing processes. The growing focus on regenerative medicine and stem cell research has further increased the need for recombinant growth factors and signaling molecules that support controlled cell differentiation and expansion. Additionally, the expansion of biopharmaceutical contract manufacturing organizations (CMOs) has accelerated the adoption of high-performance recombinant supplements to enhance production yields and maintain process consistency. The rising emphasis on 3D cell culture models and organ-on-a-chip technologies is also shaping the market, requiring advanced cell culture formulations to support complex cellular microenvironments.What Are the Key Growth Drivers of the Recombinant Cell Culture Supplements Market?
The growth in the global recombinant cell culture supplements market is driven by several factors, including increasing biopharmaceutical production, advancements in cell culture technologies, and regulatory shifts favoring serum-free and chemically defined media. The demand for monoclonal antibodies, cell-based therapies, and personalized medicine has significantly boosted the adoption of recombinant supplements in bioprocessing. The growing emphasis on reducing variability and contamination risks in cell culture has further fueled market expansion. Additionally, investments in stem cell research and tissue engineering are creating new opportunities for specialized recombinant supplements tailored for regenerative medicine applications. With continued innovation in protein expression, formulation, and bioprocess optimization, the recombinant cell culture supplements market is poised for significant growth, supporting the advancement of life sciences, pharmaceuticals, and biotechnology industries.Report Scope
The report analyzes the Recombinant Cell Culture Supplements market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Recombinant Albumin, Recombinant Insulin, Recombinant Transferrin, Recombinant Cytokines, Other Products); Application (Biopharmaceutical Production Application, Regenerative Medicines Application, Other Applications); End-Use (Pharma & Biotech Companies End-Use, Cell Culture Media Manufacturers End-Use, Contract Manufacturing Organizations & Contract Research Organizations End-Use, Contract Development & Manufacturing Organizations End-Use, Academic Research Institutes End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Some of the 36 companies featured in this Recombinant Cell Culture Supplements market report include -
- Abcam plc
- Becton, Dickinson and Company
- Bio-Techne Corporation
- Capricorn Scientific
- Corning Incorporated
- FUJIFILM Irvine Scientific Inc.
- HiMedia Laboratories
- InVitria
- Kerry Group
- Lonza Group AG
- Merck KGaA
- Miltenyi Biotec
- Novo Nordisk Pharmatech A/S
- PeproTech (acquired by Thermo Fisher Scientific)
- Repligen Corporation
- Sartorius AG
- Sino Biological Inc.
- STEMCELL Technologies Inc.
- Thermo Fisher Scientific Inc.
- Ventria Bioscience
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Recombinant Albumin segment, which is expected to reach US$818.4 Million by 2030 with a CAGR of a 10.8%. The Recombinant Insulin segment is also set to grow at 10.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $236.9 Million in 2024, and China, forecasted to grow at an impressive 11.6% CAGR to reach $281.7 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Recombinant Cell Culture Supplements Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Recombinant Cell Culture Supplements Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Recombinant Cell Culture Supplements Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Ariel Corporation, Atlas Copco, Bauer Kompressoren, BOGE Kompressoren, Burckhardt Compression and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 36 Featured):
- Abcam plc
- Becton, Dickinson and Company
- Bio-Techne Corporation
- Capricorn Scientific
- Corning Incorporated
- FUJIFILM Irvine Scientific Inc.
- HiMedia Laboratories
- InVitria
- Kerry Group
- Lonza Group AG
- Merck KGaA
- Miltenyi Biotec
- Novo Nordisk Pharmatech A/S
- PeproTech (acquired by Thermo Fisher Scientific)
- Repligen Corporation
- Sartorius AG
- Sino Biological Inc.
- STEMCELL Technologies Inc.
- Thermo Fisher Scientific Inc.
- Ventria Bioscience
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abcam plc
- Becton, Dickinson and Company
- Bio-Techne Corporation
- Capricorn Scientific
- Corning Incorporated
- FUJIFILM Irvine Scientific Inc.
- HiMedia Laboratories
- InVitria
- Kerry Group
- Lonza Group AG
- Merck KGaA
- Miltenyi Biotec
- Novo Nordisk Pharmatech A/S
- PeproTech (acquired by Thermo Fisher Scientific)
- Repligen Corporation
- Sartorius AG
- Sino Biological Inc.
- STEMCELL Technologies Inc.
- Thermo Fisher Scientific Inc.
- Ventria Bioscience
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 176 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
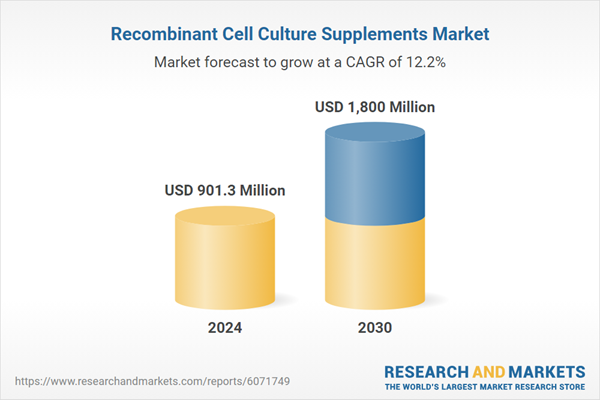
| Estimated Market Value ( USD | $ 901.3 Million |
| Forecasted Market Value ( USD | $ 1800 Million |
| Compound Annual Growth Rate | 12.2% |
| Regions Covered | Global |