Global Viral Vector Production (Research-Use) Market - Key Trends & Drivers Summarized
Why Is Viral Vector Production Critical for Biomedical Research?
Viral vector production has become a crucial component of biomedical research, particularly in the development of gene therapies, vaccines, and cancer treatments. Viral vectors serve as delivery systems for genetic material, enabling scientists to introduce, modify, or replace genes within cells to study disease mechanisms and develop targeted therapies. The increasing prevalence of genetic disorders, infectious diseases, and cancer has fueled the demand for high-quality viral vectors for preclinical and clinical research. With advancements in cell and gene therapy, researchers are leveraging viral vectors such as lentiviruses, adeno-associated viruses (AAVs), and retroviruses to develop innovative treatments for conditions that were previously considered untreatable. The ongoing development of mRNA-based vaccines and immunotherapies has further highlighted the importance of viral vector production, as researchers require scalable and efficient manufacturing processes to accelerate drug discovery and development.What Technological Innovations Are Transforming Viral Vector Production?
The field of viral vector production has seen significant advancements, improving efficiency, scalability, and safety in research applications. One of the most notable innovations is the use of suspension cell culture systems, which enable large-scale viral vector production in bioreactors, reducing costs and improving consistency. Additionally, the development of high-yield transfection reagents and optimized plasmid designs has enhanced viral vector titers, allowing researchers to produce higher-quality vectors with increased stability. Automation and AI-driven process optimization are also revolutionizing viral vector manufacturing by minimizing variability and streamlining production workflows. CRISPR-based genome editing tools are further enhancing viral vector engineering, enabling researchers to create more precise and efficient gene delivery systems. Moreover, improvements in purification technologies, such as chromatography and ultracentrifugation, have enhanced vector purity and potency, ensuring higher efficacy in gene therapy research.What Challenges Are Limiting the Expansion of Viral Vector Production?
Despite its critical role in biomedical research, viral vector production faces several challenges that impact scalability and accessibility. One of the primary obstacles is the complexity of manufacturing viral vectors, as production requires specialized cell culture facilities, biosafety protocols, and rigorous quality control measures. High production costs and limited scalability remain key barriers, particularly for smaller research institutions that lack the infrastructure to produce viral vectors in large quantities. Additionally, regulatory challenges associated with viral vector production, including safety concerns and compliance with good manufacturing practices (GMP), can slow down the research-to-market pipeline. Stability and storage limitations also pose a challenge, as some viral vectors have short shelf lives, requiring specialized storage conditions to maintain potency. Addressing these challenges requires continued investment in scalable manufacturing technologies, standardized regulatory frameworks, and cost-effective production strategies to ensure broader accessibility of viral vectors for research use.What Factors Are Driving the Growth of the Viral Vector Production (Research-Use) Market?
The growth in the viral vector production (research-use) market is driven by several factors, including the rising demand for gene therapies, advancements in vaccine development, and increasing investments in biomedical research. The growing prevalence of genetic disorders and infectious diseases has fueled the need for innovative treatment approaches, prompting researchers to develop and refine viral vector-based therapies. The expansion of personalized medicine and regenerative therapies has further accelerated the adoption of viral vectors in preclinical and clinical research. Additionally, government funding and private sector investments in biotechnology have supported the development of scalable viral vector manufacturing platforms, facilitating faster research and drug development timelines. The rapid advancement of CRISPR and genome-editing technologies has also contributed to market growth, as researchers seek efficient gene delivery tools for precision medicine applications. As demand for viral vectors continues to rise, ongoing technological advancements and process optimization efforts are expected to drive market expansion, ensuring the continued progress of gene and cell therapy research.Report Scope
The report analyzes the Viral Vector Production (Research-Use) market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Vector Type (Adeno-associated Virus, Lentivirus, Adenovirus, Retrovirus, Others); Application (Cell & Gene Therapy Development, Vaccine Development, Pharma & Biopharma Discovery, Biomedical Research); End-Use (Pharma & Biopharma Companies, Research Institutes).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Some of the 44 companies featured in this Viral Vector Production (Research-Use) market report include -
- AGC Biologics
- Batavia Biosciences
- BioNTech IMFS GmbH
- Catalent Inc.
- Cobra Biologics (Charles River Laboratories)
- FUJIFILM Diosynth Biotechnologies
- Genezen
- Lonza Group
- Merck KGaA
- Miltenyi Biotec
- Novasep Holdings SAS
- Oxford Biomedica
- REGENXBIO Inc.
- SIRION Biotech GmbH
- Takara Bio Inc.
- Thermo Fisher Scientific
- uniQure N.V.
- Virovek
- Waisman Biomanufacturing
- WuXi AppTec
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Adeno-associated Virus segment, which is expected to reach US$1.7 Billion by 2030 with a CAGR of a 17%. The Lentivirus segment is also set to grow at 16.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $472.1 Million in 2024, and China, forecasted to grow at an impressive 20.2% CAGR to reach $877.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Viral Vector Production (Research-Use) Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Viral Vector Production (Research-Use) Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Viral Vector Production (Research-Use) Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Armstrong Flooring, Beauflor, Cali Bamboo, COREtec Floors, Doma and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 44 Featured):
- AGC Biologics
- Batavia Biosciences
- BioNTech IMFS GmbH
- Catalent Inc.
- Cobra Biologics (Charles River Laboratories)
- FUJIFILM Diosynth Biotechnologies
- Genezen
- Lonza Group
- Merck KGaA
- Miltenyi Biotec
- Novasep Holdings SAS
- Oxford Biomedica
- REGENXBIO Inc.
- SIRION Biotech GmbH
- Takara Bio Inc.
- Thermo Fisher Scientific
- uniQure N.V.
- Virovek
- Waisman Biomanufacturing
- WuXi AppTec
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AGC Biologics
- Batavia Biosciences
- BioNTech IMFS GmbH
- Catalent Inc.
- Cobra Biologics (Charles River Laboratories)
- FUJIFILM Diosynth Biotechnologies
- Genezen
- Lonza Group
- Merck KGaA
- Miltenyi Biotec
- Novasep Holdings SAS
- Oxford Biomedica
- REGENXBIO Inc.
- SIRION Biotech GmbH
- Takara Bio Inc.
- Thermo Fisher Scientific
- uniQure N.V.
- Virovek
- Waisman Biomanufacturing
- WuXi AppTec
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 383 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
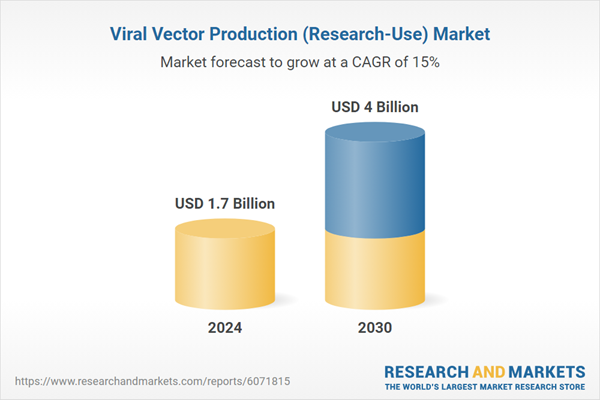
| Estimated Market Value ( USD | $ 1.7 Billion |
| Forecasted Market Value ( USD | $ 4 Billion |
| Compound Annual Growth Rate | 15.0% |
| Regions Covered | Global |