Global Virtual Clinical Trials Market - Key Trends & Drivers Summarized
How Are Virtual Clinical Trials Transforming the Drug Development Landscape?
The emergence of virtual clinical trials (VCTs) has revolutionized the traditional approach to drug development, enabling pharmaceutical companies, biotech firms, and research organizations to conduct clinical trials remotely without requiring participants to visit physical trial sites. The adoption of VCTs has significantly increased in recent years, driven by the need for faster, cost-effective, and more patient-centric trial methodologies. Unlike conventional trials, which are often hindered by geographical constraints, logistical challenges, and high dropout rates, virtual trials leverage digital technologies to streamline patient recruitment, data collection, and real-time monitoring. Mobile health (mHealth) apps, wearable biosensors, telemedicine platforms, and cloud-based data management systems have become integral to VCTs, allowing researchers to track patient health remotely and collect high-quality clinical data without physical site visits. The shift toward decentralized clinical trials has been accelerated by the COVID-19 pandemic, which underscored the limitations of in-person trial models and highlighted the potential of remote trial methodologies. With regulatory agencies increasingly endorsing digital trial frameworks and pharmaceutical companies investing heavily in virtual trial platforms, VCTs are poised to become the standard for modern clinical research, offering unprecedented flexibility and accessibility for participants worldwide.What Technological Innovations Are Driving the Adoption of Virtual Clinical Trials?
The success of virtual clinical trials is largely attributed to rapid advancements in digital health technologies, artificial intelligence (AI), and real-time data analytics. One of the most transformative innovations in VCTs is the integration of AI-driven patient recruitment tools, which use predictive algorithms to identify suitable candidates based on medical history, genetic data, and lifestyle factors. These technologies significantly reduce recruitment timelines and enhance patient diversity, addressing long-standing challenges in clinical research. Wearable medical devices and remote monitoring tools have also revolutionized VCTs by enabling continuous tracking of vital signs, medication adherence, and disease progression in real-world settings. Blockchain technology is further enhancing data security and transparency in virtual trials, providing immutable and tamper-proof clinical records while ensuring compliance with regulatory standards. Additionally, the rise of cloud-based clinical trial management systems (CTMS) has streamlined data storage, analysis, and collaboration between stakeholders, improving trial efficiency and scalability. The integration of telemedicine and video conferencing has also facilitated direct interactions between trial participants and investigators, reducing the need for physical visits while maintaining high levels of patient engagement. These technological advancements are not only improving trial efficiency but also enhancing data accuracy, regulatory compliance, and overall patient experience in the clinical research process.What Challenges Are Hindering the Expansion of Virtual Clinical Trials?
Despite their transformative potential, virtual clinical trials face several challenges that could hinder their widespread adoption. One of the most pressing issues is regulatory compliance, as different countries have varying guidelines and ethical considerations regarding digital health technologies and remote patient monitoring. Ensuring data privacy and security is another major concern, as VCTs rely heavily on digital platforms that store sensitive patient information, making them vulnerable to cybersecurity threats and data breaches. Additionally, the digital divide poses a significant barrier to virtual trial accessibility, as not all patients have access to high-speed internet, smartphones, or wearable devices required for participation. Another challenge lies in ensuring patient adherence and engagement throughout the trial period, as remote participation may lead to reduced interaction between patients and trial coordinators, impacting data quality and retention rates. Pharmaceutical companies and research organizations must also address concerns related to data standardization, interoperability between different digital health platforms, and the validation of remote monitoring tools to ensure the accuracy and reliability of collected clinical data. Overcoming these challenges will require a collaborative effort between regulatory agencies, technology providers, and healthcare institutions to establish standardized frameworks and best practices for conducting virtual clinical trials effectively.What Factors Are Driving the Growth of the Virtual Clinical Trials Market?
The growth in the virtual clinical trials market is driven by several factors, including the increasing adoption of digital health technologies, rising demand for decentralized clinical research, and the need for cost-effective and time-efficient trial methodologies. The growing prevalence of chronic diseases and rare conditions has necessitated innovative trial approaches that can accelerate drug development while ensuring diverse patient representation. The expansion of AI-driven patient recruitment and real-time monitoring solutions has also contributed to market growth, enabling researchers to conduct large-scale trials with enhanced efficiency. The increasing support from regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for decentralized trials has further bolstered industry adoption, as companies seek to align with evolving compliance requirements. Additionally, the surge in investments from pharmaceutical companies and contract research organizations (CROs) in virtual trial infrastructure is driving market expansion, with industry players focusing on AI-powered data analytics, blockchain-based security solutions, and cloud-based clinical trial platforms. The growing emphasis on patient-centric trial designs, along with the rising adoption of wearable health monitoring devices, is expected to further propel the virtual clinical trials market, positioning it as a key pillar in the future of drug development and clinical research.Report Scope
The report analyzes the Virtual Clinical Trials market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Study Design (Interventional, Observational, Expanded Access); Indication (CNS, Autoimmune / Inflammation, Cardiovascular Disease, Metabolic / Endocrinology, Infectious Disease, Oncology, Genitourinary, Ophthalmology, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Some of the 47 companies featured in this Virtual Clinical Trials market report include -
- Castor
- Clinical Ink Inc.
- Covance Inc. (LabCorp)
- CRF Health
- ICON plc
- IQVIA
- LEO Innovation Lab
- Medable Inc.
- Medidata Solutions (Dassault Systèmes SE)
- Medpace Holdings Inc.
- Novotech
- Oracle Corporation
- Parexel International Corporation
- PRA Health Sciences
- Science 37
- Signant Health
- Syneos Health
- THREAD Research
- Velocity Clinical Research
- Wuxi AppTec
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Interventional Study segment, which is expected to reach US$7.5 Billion by 2030 with a CAGR of a 5.5%. The Observational Study segment is also set to grow at 6.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.6 Billion in 2024, and China, forecasted to grow at an impressive 5.7% CAGR to reach $2.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Virtual Clinical Trials Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Virtual Clinical Trials Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Virtual Clinical Trials Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Amazon Web Services, Inc., Cisco Systems, Inc., Citrix Systems, Inc., Dell Inc., Ericom Software and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 47 Featured):
- Castor
- Clinical Ink Inc.
- Covance Inc. (LabCorp)
- CRF Health
- ICON plc
- IQVIA
- LEO Innovation Lab
- Medable Inc.
- Medidata Solutions (Dassault Systèmes SE)
- Medpace Holdings Inc.
- Novotech
- Oracle Corporation
- Parexel International Corporation
- PRA Health Sciences
- Science 37
- Signant Health
- Syneos Health
- THREAD Research
- Velocity Clinical Research
- Wuxi AppTec
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Castor
- Clinical Ink Inc.
- Covance Inc. (LabCorp)
- CRF Health
- ICON plc
- IQVIA
- LEO Innovation Lab
- Medable Inc.
- Medidata Solutions (Dassault Systèmes SE)
- Medpace Holdings Inc.
- Novotech
- Oracle Corporation
- Parexel International Corporation
- PRA Health Sciences
- Science 37
- Signant Health
- Syneos Health
- THREAD Research
- Velocity Clinical Research
- Wuxi AppTec
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 159 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
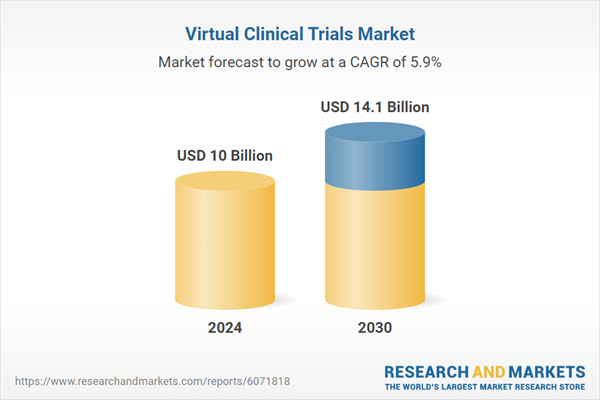
| Estimated Market Value ( USD | $ 10 Billion |
| Forecasted Market Value ( USD | $ 14.1 Billion |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | Global |