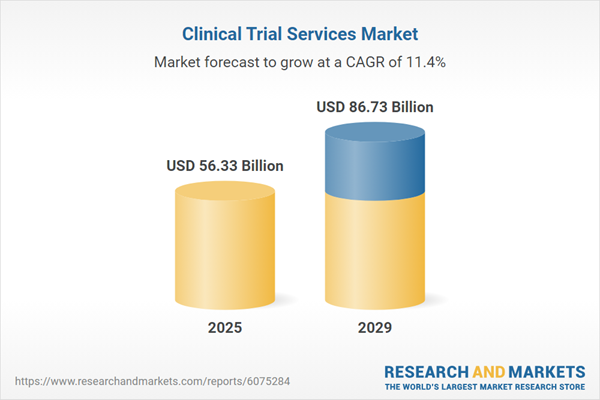
The clinical trial services market size has grown rapidly in recent years. It will grow from $50.45 billion in 2024 to $56.33 billion in 2025 at a compound annual growth rate (CAGR) of 11.66%. The growth during the historical period can be attributed to factors such as the growing demand for personalized medicine, an increase in the outsourcing of clinical trials, higher healthcare spending, greater globalization, and expanded government initiatives.
The clinical trial services market size is expected to see rapid growth in the next few years. It will grow to $86.73 billion in 2029 at a compound annual growth rate (CAGR) of 11.40%. The projected growth in the forecast period is driven by the rising incidence of chronic diseases, increased investments in pharmaceutical research and development, the expanding geriatric population, a growing number of clinical trials, and higher investments in the development of advanced drugs. Key trends expected in the forecast period include advancements in technology, the use of artificial intelligence and machine learning, virtual clinical trials, and the continued evolution of personalized medicine.
The increasing demand for personalized medicine is expected to drive the growth of the clinical trial services market. Personalized medicine is a healthcare approach that tailors treatments to individual patients based on factors such as their genetic makeup, environment, and lifestyle. The demand for personalized medicine is rising due to advancements in genomic technologies, the emergence of targeted therapies, patients' growing desire for customized treatments, and the integration of artificial intelligence, biomarkers, and real-world data to enhance treatment efficacy and safety. Clinical trial services play an essential role in advancing personalized medicine by supporting the development, testing, and validation of targeted therapies using genetic data, biomarkers, and personalized treatment protocols, which ensures more effective and individualized patient outcomes. For example, in February 2024, the Personalized Medicine Coalition, a U.S.-based organization, reported that the FDA approved 16 new personalized treatments for rare disease patients in 2023, a significant increase from six approvals in 2022. This growing demand for personalized medicine is contributing to the expansion of the clinical trial services market.
Companies in the clinical trial services market are developing data solutions to accelerate the clinical trial process. These solutions include technologies and tools designed to manage, analyze, and optimize data to improve efficiency, accuracy, and decision-making in clinical trials. For example, in July 2024, Laboratory Corporation, a U.S.-based life sciences company, introduced Labcorp Global Trial Connect, a comprehensive set of central laboratory solutions designed to streamline and improve the clinical trial process. This integrated platform connects central laboratories, clinical trial sites, and study sponsors, enabling seamless communication and real-time data sharing, which helps reduce delays and improve trial timelines. It also enhances patient access by using advanced technologies to identify suitable participants and facilitate remote monitoring.
In February 2024, ICON plc, an Ireland-based clinical research company, acquired Clinical Research Management Inc. for an undisclosed amount. This acquisition enables ICON to enhance its clinical trial services by expanding its clinical research management expertise, improving operational efficiency, and offering a broader range of solutions to its clients. Clinical Research Management Inc., a U.S.-based company, specializes in providing services within the clinical research field.
Major players in the clinical trial services market are Accenture plc., IQVIA Holdings Inc., Laboratory Corporation of America Holdings, ICON Public Limited Company, WuXi AppTec Co. Ltd., Charles River Laboratories International Inc, Parexel International Corporation, Medpace Holdings Inc, BioClinica Inc, Worldwide Clinical Trials Inc, Medidata Solutions Inc, Precision Medicine Group Inc, Celerion Inc, MakroCare Clinical Research Limited, PCI Pharma Services, Ecron Acunova Limited, PPD LLC., Clinipace Inc., Syntactx LLC., Veristat LLC., AutoCruitment Inc.
North America was the largest region in the clinical trial services market in 2024. North America is expected to be the fastest-growing region in the forecast period. The regions covered in clinical trial services report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the clinical trial services market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Clinical trial services encompass a wide range of activities and support throughout the stages of designing, conducting, and analyzing clinical trials. These services are crucial for evaluating the safety, effectiveness, and regulatory adherence of new medical treatments, drugs, or devices. They cover various aspects such as patient recruitment, data collection, monitoring, regulatory submissions, statistical analysis, and final reporting, ensuring that clinical studies follow regulatory standards and contribute to medical advancements.
The primary service categories in clinical trial services include clinical trial management (CTM), planning and design, project management, monitoring and data management, site management, regulatory services, regulatory submission, consulting, and protocol and safety consulting. Clinical trial management (CTM) focuses on overseeing all facets of a trial, such as scheduling, resource allocation, regulatory compliance, and ensuring smooth operations. Clinical trials progress through phases, Phase I, Phase II, Phase III, and Phase IV. They also cover therapeutic areas such as oncology, cardiology, neurology, infectious diseases, immunology, respiratory, and dermatology. The main users of clinical trial services are pharmaceutical and biotechnology companies, medical device firms, contract research organizations, and academic and research institutions.
The clinical trial services market research report is one of a series of new reports that provides clinical trial services market statistics, including clinical trial services industry global market size, regional shares, competitors with a clinical trial services market share, detailed clinical trial services market segments, market trends and opportunities, and any further data you may need to thrive in the clinical trial services industry. This clinical trial services market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The clinical trial services market includes revenues earned by entities by project management, clinical trial documentation, and safety monitoring. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the provision of clinical trial services within the specified market and geography through service contracts, grants, or partnerships in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where the services are provided. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other services.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Clinical Trial Services Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on clinical trial services market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for clinical trial services? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The clinical trial services market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) by Service Type: Clinical Trial Management (CTM); Planning and Design; Project Management; Monitoring and Data Management; Site Management; Regulatory Services; Regulatory Submission; Consulting; Protocol and Safety Consulting; Other Service Types2) by Phase: Phase I; Phase II; Phase III; Phase IV
3) by Therapeutic Area: Oncology; Cardiology; Neurology; Infectious Diseases; Immunology; Respiratory; Dermatology
4) by End-User: Pharmaceutical and Biotechnology Companies; Medical Device Companies; Contract Research Organizations; Academic and Research Institutes
Subsegments:
1) by Clinical Trial Management (CTM): Study Setup and Execution; Recruitment and Patient Enrollment; Trial Monitoring and Reporting; Trial Close-Out and Data Lock2) by Planning and Design: Protocol Development; Clinical Trial Design; Feasibility Analysis; Site Selection and Initiation
3) by Project Management: Budgeting and Resource Allocation; Risk Management; Timeline Management; Stakeholder Coordination
4) by Monitoring and Data Management: Data Collection and Validation; Statistical Analysis; Quality Control and Assurance; Data Reporting and Interpretation
5) by Site Management: Site Initiation and Training; Site Monitoring and Audits; Site Close-Out and Documentation
6) by Regulatory Services: Regulatory Compliance and Consultation; Regulatory Strategy Development; Preparation For Audits and Inspections
7) by Regulatory Submission: Submission of Investigational New Drug (IND) Applications; New Drug Application (NDA) Submission; Submission of Clinical Study Reports (CSRs)
8) by Consulting: Strategy and Planning Consulting; Operational and Performance Consulting; Data Analytics and Interpretation Consulting
9) by Protocol and Safety Consulting: Clinical Trial Protocol Consulting; Safety Management and Risk Assessment; Safety Reporting and Adverse Event Management
10) by Other Service Types: Medical Writing; Biostatistics and Data Analysis Services; Patient Recruitment Services; Post-Approval Studies.
Key Companies Profiled: Accenture plc.; IQVIA Holdings Inc.; Laboratory Corporation of America Holdings; ICON Public Limited Company; WuXi AppTec Co. Ltd.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Clinical Trial Services market report include:- Accenture plc.
- IQVIA Holdings Inc.
- Laboratory Corporation of America Holdings
- ICON Public Limited Company
- WuXi AppTec Co. Ltd.
- Charles River Laboratories International Inc
- Parexel International Corporation
- Medpace Holdings Inc
- BioClinica Inc
- Worldwide Clinical Trials Inc
- Medidata Solutions Inc
- Precision Medicine Group Inc
- Celerion Inc
- MakroCare Clinical Research Limited
- PCI Pharma Services
- Ecron Acunova Limited
- PPD LLC.
- Clinipace Inc.
- Syntactx LLC.
- Veristat LLC.
- AutoCruitment Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 175 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 56.33 Billion |
| Forecasted Market Value ( USD | $ 86.73 Billion |
| Compound Annual Growth Rate | 11.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |