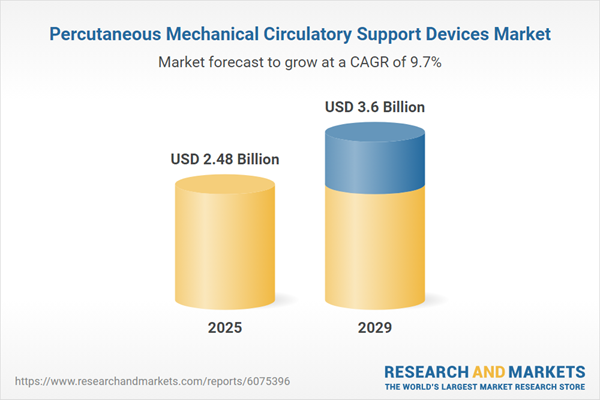
The percutaneous mechanical circulatory support devices market size has grown rapidly in recent years. It will grow from $2.26 billion in 2024 to $2.48 billion in 2025 at a compound annual growth rate (CAGR) of 10%. The growth during the historic period can be attributed to factors such as the rising incidence of cardiovascular diseases, a growing geriatric population, an increasing number of heart attacks, heightened awareness and screening programs, and a growing demand for heart pump devices.
The percutaneous mechanical circulatory support devices market size is expected to see strong growth in the next few years. It will grow to $3.6 billion in 2029 at a compound annual growth rate (CAGR) of 9.7%. The growth during the forecast period can be attributed to factors such as the increasing demand for long-term mechanical circulatory support, rising healthcare expenditure, a growing diabetic and obese population, a higher demand for heart transplants, and greater acceptance of percutaneous procedures. Key trends expected to drive growth include advancements in pump technology, innovations in medical and cardiac support technologies, the rise of minimally invasive medical technologies, and the development of biocompatible materials.
The growing prevalence of cardiovascular diseases is expected to drive the expansion of the percutaneous mechanical circulatory support devices market. Cardiovascular diseases (CVDs) encompass a range of disorders affecting the heart and blood vessels, including conditions such as coronary artery disease, heart attacks, heart failure, stroke, and high blood pressure. The rise in cardiovascular diseases can be attributed to factors such as an aging population, poor lifestyle choices, obesity, hypertension, and genetic factors. Percutaneous mechanical circulatory support devices are used to enhance cardiac function, alleviate heart strain, and aid recovery in severe heart conditions. For example, in December 2023, the Australian Institute of Health and Welfare, a government agency in Australia, reported that deaths from coronary heart disease (CHD) increased from 14,100 in 2021 to 14,900 in 2022. As a result, the growing incidence of cardiovascular diseases is fueling the growth of the percutaneous mechanical circulatory support (MCS) devices market.
Leading companies in the percutaneous mechanical circulatory support devices market are concentrating on developing advanced solutions, such as intra-aortic axial-flow devices, to improve patient outcomes and reduce surgical risks. An intra-aortic axial-flow device is a type of percutaneous mechanical circulatory support (MCS) device designed to support heart function by increasing blood flow from the left ventricle to the aorta. For example, in February 2023, Procyrion, Inc., a US-based medical device company, announced the successful first-in-human use of its Aortix pump. This innovative device aims to prevent acute kidney injury (AKI) in patients undergoing cardiac surgery. The Aortix pump, delivered via a catheter into the descending thoracic aorta, enhances renal perfusion while reducing the heart's workload during surgery. By improving both renal and systemic perfusion and decreasing heart strain, the device is particularly beneficial for patients with impaired left ventricular function and renal issues. Capable of providing up to 5 liters of flow per minute, the Aortix pump is intended for short-term use during procedures such as percutaneous coronary interventions (PCI).
In December 2022, Johnson & Johnson Private Limited, a pharmaceutical company based in the US, acquired Abiomed for $16.6 billion. This acquisition is part of Johnson & Johnson's strategy to expand its cardiovascular medical technology offerings, advance innovative heart recovery solutions, and reinforce its position as a global leader in the MedTech sector. Abiomed Inc., a US-based medical device company, is known for producing percutaneous mechanical circulatory support (PMCS) devices, particularly the Impella series.
Major players in the percutaneous mechanical circulatory support devices market are Abbott Laboratories, Medtronic Plc, Getinge AB, Teleflex Incorporated, Biotronik, Abiomed Inc., LivaNova Plc, Bioventrix, Berlin Heart, Eurosets, Magenta Medical Ltd, SynCardia Systems LLC, CardiacAssist Inc., Jarvik Heart Inc., Procyrion, BiVACOR Inc., CardioBridge GmbH and Company, Circulite Medical Inc., NovaPump GmbH, Supira Medical Inc., and Pulsatile Technologies Inc.
North America was the largest region in the percutaneous mechanical circulatory support devices market in 2024. The regions covered in percutaneous mechanical circulatory support (MCS) devices report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the percutaneous mechanical circulatory support (MCS) devices market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Percutaneous mechanical circulatory support (pMCS) devices are minimally invasive systems designed to temporarily assist or fully replace the heart's pumping function. These devices are inserted through a catheter into blood vessels, typically via the femoral artery, and positioned within the heart to provide mechanical support during acute heart failure, cardiogenic shock, or high-risk cardiac interventions.
The main types of percutaneous mechanical circulatory support devices include intra-aortic balloon pumps, Impella devices, extracorporeal membrane oxygenation, TandemHeart, and other variations. An intra-aortic balloon pump (IABP) is a device that helps the heart pump blood by inserting a balloon catheter into the aorta, the large artery carrying blood from the heart. It is used to support patients with severe cardiac conditions such as cardiogenic shock, acute myocardial infarction, and heart failure, among others. IABPs are utilized by various end users, including hospitals, ambulatory surgical centers, and other healthcare settings.
The percutaneous mechanical circulatory support devices market research report is one of a series of new reports that provides percutaneous mechanical circulatory support devices market statistics, including the percutaneous mechanical circulatory support devices industry global market size, regional shares, competitors with the percutaneous mechanical circulatory support devices market share, detailed percutaneous mechanical circulatory support devices market segments, market trends, and opportunities, and any further data you may need to thrive in the percutaneous mechanical circulatory support devices industry. This percutaneous mechanical circulatory support devices market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The percutaneous mechanical circulatory support (MCS) devices market consists of sales of left ventricular assist devices, centrifugal flow pumps, and percutaneous right ventricular assist devices. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Percutaneous Mechanical Circulatory Support Devices Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on percutaneous mechanical circulatory support devices market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for percutaneous mechanical circulatory support devices? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The percutaneous mechanical circulatory support devices market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) by Product Type: Intra-Aortic Balloon Pumps; Impella Devices; Extracorporeal Membrane Oxygenation; TandemHeart; Other Types2) by Application: Cardiogenic Shock; Acute Myocardial Infarction; Heart Failure; Other Applications
3) by End-User: Hospitals; Ambulatory Surgical Centers; Other End Users
Subsegments:
1) by Intra-Aortic Balloon Pumps (IABP): Standard IABP Devices; Enhanced IABP Devices2) by Impella Devices: Impella 2.5; Impella 5.0; Impella CP (Cardiac Power)
3) by Extracorporeal Membrane Oxygenation (ECMO): Veno-Venous ECMO (VV ECMO); Veno-Arterial ECMO (VA ECMO)
4) by Tandemheart: Tandemheart PUMP System; Tandemheart PRO System
5) by Other Types: Centrifugal Pumps; Pneumatic Assist Devices
Key Companies Profiled: Abbott Laboratories; Medtronic Plc; Getinge AB; Teleflex Incorporated; Biotronik
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Percutaneous Mechanical Circulatory Support Devices market report include:- Abbott Laboratories
- Medtronic Plc
- Getinge AB
- Teleflex Incorporated
- Biotronik
- Abiomed Inc.
- LivaNova Plc
- Bioventrix
- Berlin Heart
- Eurosets
- Magenta Medical Ltd
- SynCardia Systems LLC
- CardiacAssist Inc.
- Jarvik Heart Inc.
- Procyrion
- BiVACOR Inc.
- CardioBridge GmbH and Company
- Circulite Medical Inc.
- NovaPump GmbH
- Supira Medical Inc.
- Pulsatile Technologies Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 175 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.48 Billion |
| Forecasted Market Value ( USD | $ 3.6 Billion |
| Compound Annual Growth Rate | 9.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |