Speak directly to the analyst to clarify any post sales queries you may have.
Seafood microbiological detection is becoming a strategic control point as global supply chains, audits, and consumer expectations intensify
Seafood sits at the intersection of nutrition, global trade, and public health, which makes microbiological detection a board-level priority rather than a back-of-house laboratory task. From aquaculture ponds and harvest vessels to processing lines and retail cold chains, products often experience multiple handoffs, variable temperature exposure, and diverse water sources. Each step can introduce or amplify microbial risks, including pathogenic bacteria, viruses, and indicator organisms that signal sanitation or process-control failures.In response, microbiological detection in seafood is evolving from periodic compliance sampling to a more continuous, risk-informed discipline. Companies increasingly treat testing as an operational control that verifies preventive measures, validates cleaning effectiveness, and supports rapid decision-making when deviations occur. This shift is reinforced by tighter buyer specifications, more frequent audits, and heightened consumer expectations for transparency in how food safety is managed.
At the same time, the detection toolbox is expanding. Traditional culture-based methods still anchor confirmatory testing and regulatory acceptance, but they are being complemented by immunoassays and molecular techniques that provide faster time-to-answer. As laboratories and plant quality teams modernize, they also must navigate method validation, matrix effects specific to seafood, competency requirements, and data integrity standards that determine whether results can stand up to scrutiny.
Against this backdrop, the executive summary that follows examines the forces reshaping seafood microbiological detection, the implications of changing trade policy, and the practical segmentation perspectives that clarify where demand is accelerating and why. It concludes with targeted recommendations to help stakeholders strengthen assurance, reduce response time, and build more resilient testing strategies.
Rapid methods, digital traceability, and value-chain verification are redefining how seafood microbiology programs prove control and speed decisions
The landscape is being transformed first by a clear acceleration toward faster, decision-grade results. Seafood processors and importers are increasingly aligning testing cadence with operational risk, which places a premium on methods that shorten hold times and enable earlier intervention. As a result, rapid screening approaches are being adopted more broadly, while classical microbiology is being repositioned for confirmation, investigations, and root-cause analysis. This is not a replacement story so much as a workflow redesign, where speed and defensibility are optimized together.A second shift is the movement from single-point testing to programmatic verification across the value chain. Rather than relying solely on finished-product tests, more organizations are expanding environmental monitoring in high-care zones, strengthening water and ice testing, and using supplier verification as a proactive barrier. This reflects the reality that many seafood hazards are better managed through process control and hygienic design than through end-product sampling alone.
Digitalization is also changing what “good” looks like. Laboratories and quality teams are integrating instruments with laboratory information management systems and quality management platforms to improve traceability, streamline chain-of-custody, and reduce transcription errors. This matters because microbiological results are increasingly used not only to release product, but also to demonstrate preventive control effectiveness, respond to customer queries, and support corrective actions that require auditable documentation.
Method performance expectations are rising as well. Seafood matrices can be complex due to salt content, fats, and naturally occurring microflora that may inhibit amplification or complicate enrichment. Consequently, there is greater emphasis on matrix-specific validation, robust sample preparation, and use of controls that detect inhibition or cross-contamination. These technical considerations are becoming procurement criteria, not just laboratory preferences.
Finally, the sector is seeing heightened scrutiny around antimicrobial resistance and emerging pathogens, along with renewed focus on viral risks in bivalves and ready-to-eat products. While routine programs still prioritize established targets, forward-looking companies are building the capability to adapt panels, update methods, and respond quickly when scientific guidance or buyer requirements change. Collectively, these shifts are driving a more integrated, faster, and more accountable microbiological detection ecosystem in seafood.
US tariffs in 2025 may reshape sourcing and margins, increasing the need for agile verification, matrix-fit methods, and risk-based testing design
United States tariffs in 2025 are expected to influence seafood microbiological detection primarily through cost structures, sourcing decisions, and compliance posture. When tariffs raise landed costs for certain categories of seafood or inputs, companies often respond by diversifying suppliers, shifting product mix, or re-routing through different processing geographies. Each of these adjustments can introduce new microbiological risk variables, including unfamiliar sanitation practices, different water quality profiles, and changes in cold chain reliability.In practice, supply chain reconfiguration increases the need for enhanced supplier verification and more frequent receiving tests, especially during onboarding phases. Importers and processors may tighten acceptance criteria, require additional certificates of analysis, or demand method transparency from upstream laboratories. However, relying solely on paperwork can be fragile; consequently, many organizations reinforce their own inbound testing to confirm that documentation matches real-world microbial performance.
Tariff-related cost pressure can also create tension between speed and expense. Rapid molecular workflows typically reduce holding time and may prevent costly recalls, but they can require higher per-test consumable costs and specialized training. Under margin compression, some firms may be tempted to reduce testing frequency or revert to slower methods. The more resilient approach is to optimize programs based on risk, using rapid screening where time-to-release is critical and reserving culture confirmation strategically, thereby controlling total cost of quality rather than just the laboratory line item.
Another cumulative impact is operational: changes in supplier mix can alter the baseline microflora in raw materials, which in turn affects enrichment dynamics, false-positive rates, and the interpretability of indicator organisms. Laboratories may need to recalibrate workflows, revisit sampling plans, and ensure that methods remain fit-for-purpose for new species or product forms.
Finally, tariffs can catalyze domestic investment in processing or value-added steps, which expands the number of facilities that must maintain validated testing programs. As more operations take on higher-risk handling activities-such as thawing, marinating, smoking, or ready-to-eat packaging-the detection strategy must evolve accordingly, with stronger environmental monitoring, faster investigative testing, and clearer corrective-action triggers. Overall, the 2025 tariff environment is likely to increase the premium on agility: companies that can qualify new suppliers quickly, validate methods across matrices, and document control will be better positioned to manage both economic and microbiological uncertainty.
Segmentation reveals where risk, speed, and method choice diverge across seafood types, target organisms, technologies, sample matrices, and end users
Segmentation clarifies how buyers prioritize speed, confidence, and operational fit across different use cases. By product type, seafood microbiological detection requirements diverge meaningfully: finfish often emphasizes control of bacterial pathogens associated with handling and temperature abuse, while crustaceans and cephalopods can present distinct spoilage dynamics and sanitation sensitivities during processing. Bivalves add a separate risk profile where viral contamination and water quality play an outsized role, making upstream monitoring and lot traceability especially consequential.By target organism, programs typically balance pathogen detection with indicators that reflect process hygiene. Pathogen-focused testing is commonly anchored on Salmonella, Listeria monocytogenes, and Vibrio species, with emphasis shaped by product format and whether items are ready-to-eat. In parallel, indicator organisms such as total viable count, coliforms, and E. coli support trend analysis and sanitation verification, helping plants detect drift before it becomes a release-blocking failure.
By technology, demand is splitting into complementary lanes. Culture-based methods remain central for confirmation and regulatory defensibility, particularly when enforcement actions or customer disputes require gold-standard evidence. Immunoassays are often used as practical screening tools in high-throughput settings, while PCR and other nucleic acid amplification approaches are increasingly chosen for rapid turnaround and sensitivity. Sequencing and advanced typing are gaining relevance for investigation and source tracking, especially when companies need to distinguish persistent environmental niches from one-time events.
By sample type, the industry is steadily shifting from an overreliance on finished-product testing toward broader coverage that includes environmental swabs, processing water, ice, and contact surfaces. This change reflects the operational reality that detecting contamination early in the environment can prevent widespread product exposure and reduce rework. The specific mix depends on facility design, product flow, and the degree of post-lethality exposure.
By testing location and end user, the decision calculus changes again. In-house laboratories prioritize control, speed, and integration with production schedules, whereas third-party laboratories emphasize accreditation scope, surge capacity, and geographic proximity to ports and processing hubs. Processors, importers, retailers, and foodservice buyers also weigh risk differently; for example, retailers may emphasize standardization and audit readiness across suppliers, while processors may focus on line-side decisions that protect throughput.
By workflow and throughput requirements, high-volume operations tend to favor automation, streamlined sample preparation, and digital result capture to reduce human variability. Smaller producers may adopt more targeted testing plans but still seek methods that reduce hold times and improve confidence. Across segments, the strongest programs align method selection with the decision being made-release, investigation, verification, or trend control-so that each test delivers actionable value rather than simply generating data.
Regional dynamics shape testing priorities as trade exposure, lab capacity, and buyer standards vary across the Americas, Europe, Middle East & Africa, and Asia-Pacific
Regional dynamics in seafood microbiological detection reflect differences in regulatory emphasis, dominant species, infrastructure maturity, and trade exposure. In the Americas, large import volumes and diverse sourcing create strong demand for standardized inbound verification, rapid release decisions, and robust documentation that can withstand customer and regulatory scrutiny. The presence of large-scale processing and distribution networks also increases the need for consistent environmental monitoring and data systems that support multi-site governance.In Europe, a strong culture of food safety management systems and rigorous retailer specifications reinforces method validation, accreditation discipline, and harmonized testing approaches across complex supply networks. Cross-border trade within the region adds practical urgency for comparable results and traceable workflows, particularly for ready-to-eat and cold-smoked products where Listeria control is a prominent concern. As sustainability and provenance expectations rise, microbiological programs are increasingly expected to complement traceability narratives with demonstrable preventive controls.
The Middle East and Africa show a dual reality of rapidly expanding cold chain capabilities alongside varied laboratory capacity and regulatory maturity. In many markets, modernization of ports, logistics, and retail is elevating expectations for microbiological assurance, especially for imported seafood. This contributes to growing reliance on capable third-party laboratories near key entry points, coupled with greater interest in portable or rapid solutions where infrastructure constraints make traditional testing slower or less consistent.
Asia-Pacific remains central to global seafood production and processing, which creates a broad spectrum of needs-from high-throughput export facilities that require internationally accepted methods to domestic channels where modernization is accelerating. Export-oriented operations often invest in rapid screening and strong documentation to meet destination requirements, while regional diversification of aquaculture species and processing formats drives demand for methods proven across multiple matrices. Additionally, the region’s scale makes automation and workforce training critical, as high sample volumes can strain manual workflows and increase variability.
Across all regions, one shared trend stands out: buyers increasingly expect comparability and defensibility of results, regardless of where testing occurs. This is pushing laboratories and processors toward clearer method governance, stronger proficiency programs, and data integrity practices that can support rapid decisions without sacrificing credibility. Regional differences persist, but the direction of travel is consistent-toward faster, more integrated, and more auditable microbiological detection programs.
Competitive advantage hinges on validated performance in seafood matrices, integrated workflows, strong support models, and investigation-grade characterization capabilities
Key companies in seafood microbiological detection compete on method credibility, workflow efficiency, and the ability to translate laboratory results into operational decisions. Leaders in instruments and assays differentiate through turnaround time, sensitivity and specificity in challenging seafood matrices, and the breadth of validated applications across species and product forms. Just as important, they compete on how reliably their systems perform in real-world settings where enrichment conditions, inhibitors, and background flora can complicate outcomes.A notable area of competition is end-to-end workflow integration. Vendors that pair sample preparation solutions with robust assays and user-friendly software reduce the friction that often delays adoption. Increasingly, customers evaluate not only the assay but also the ease of training, maintenance requirements, quality controls, and compatibility with laboratory information systems. This is especially relevant for multi-site processors and third-party labs that must standardize practices while managing staff turnover and variable sample loads.
Service and support models are also becoming differentiators. Accreditation readiness, method verification guidance, and troubleshooting assistance matter when laboratories are under pressure to defend results during audits or investigations. Companies that provide strong technical documentation, clear validation pathways, and responsive field support often earn long-term loyalty, particularly in environments where downtime can disrupt production schedules.
Another competitive frontier is advanced characterization for investigations. While routine testing focuses on presence/absence and indicator counts, episodic events require deeper insight into routes of contamination and persistence in facilities. Providers that enable typing, trend analytics, and investigation workflows-while maintaining data integrity-help customers shorten root-cause timelines and reduce recurrence.
Finally, partnerships across the ecosystem are expanding. Instrument suppliers, reagent developers, contract laboratories, and digital platform providers increasingly collaborate to deliver bundled solutions that address throughput, traceability, and compliance requirements together. As customers seek fewer handoffs and clearer accountability, this integrated approach is likely to shape procurement decisions and accelerate modernization across the sector.
Leaders can reduce risk and cost-of-quality by aligning tests to decisions, standardizing governance, digitizing workflows, and qualifying suppliers proactively
Industry leaders can strengthen microbiological assurance by first aligning testing strategy to decision points rather than habit. This means explicitly mapping which tests support product release, which verify sanitation, which qualify suppliers, and which drive investigations. When these decision points are clear, organizations can justify rapid methods for time-sensitive release, maintain culture confirmation for defensibility, and avoid over-testing in low-risk contexts.Next, invest in method governance that travels across sites and suppliers. Standardized sampling plans, defined acceptance criteria, and documented corrective-action triggers reduce variability and ensure that results are comparable over time. Where supply chains are changing due to trade or cost pressures, tighten onboarding protocols for new suppliers by combining documentation reviews with targeted verification testing and early-life trend monitoring.
Operationally, shorten response time by integrating testing with digital traceability and structured data review. Connecting instruments or results portals to quality systems enables faster holds and releases, quicker escalation, and better trending of indicator organisms that can reveal early drift. In parallel, strengthen environmental monitoring in zones most associated with cross-contamination and post-process exposure, and ensure cleaning validation includes both ATP-style checks where appropriate and microbiological verification for higher-risk areas.
Capability building is equally important. Train teams not only on running assays, but also on interpreting results in the context of seafood-specific matrix effects and enrichment behavior. Establish routine proficiency checks, clear contamination-control practices, and periodic verification of methods when product mix or suppliers change. These steps reduce false alarms and ensure that rapid methods deliver credible, audit-ready outcomes.
Finally, treat tariffs and broader geopolitical uncertainty as a trigger for resilience planning. Develop dual sourcing strategies that include microbiological qualification criteria, maintain surge capacity with third-party labs for peak periods, and pre-define escalation pathways for atypical results. Organizations that can adapt quickly without diluting standards will protect brand trust while maintaining operational continuity.
A decision-oriented methodology blends stakeholder interviews, value-chain risk mapping, and technical triangulation to reflect real seafood testing workflows
The research methodology for this executive summary is built to reflect how seafood microbiological detection decisions are made in practice, combining technical evaluation with operational context. The approach begins with structured analysis of the seafood value chain, identifying where microbial risks concentrate across harvest, transport, processing, and distribution, and mapping which testing workflows are used to control those risks. This establishes a practical foundation for interpreting why certain methods and deployments are favored.Primary research is conducted through interviews and consultations with stakeholders across the ecosystem, including quality and food safety leaders at seafood processors, importers, and retail supply organizations, along with laboratory managers, auditors, and technology providers. These conversations focus on workflow pain points, method selection criteria, validation expectations, turnaround requirements, and adoption barriers such as staffing, training, and integration with digital systems.
Secondary research complements these insights by reviewing regulatory guidance, standards relevant to food microbiology programs, published scientific literature on seafood matrices and pathogen behavior, and publicly available information from technology providers and accredited laboratories. This helps triangulate claims about method performance, typical workflows, and evolving compliance expectations without relying on any single viewpoint.
The study applies segmentation logic to organize findings across seafood product types, target organisms, testing technologies, sample types, and end-user settings. Qualitative cross-comparisons are then used to identify consistent themes, points of divergence, and adoption triggers, such as the shift toward environmental monitoring or the operational value of reduced hold times.
Finally, quality control is maintained through internal consistency checks, terminology standardization, and peer review of interpretations to ensure conclusions are technically grounded and aligned with current industry practices. The result is a decision-oriented view of the seafood microbiological detection landscape that emphasizes actionable understanding over abstract description.
Seafood microbiological detection is evolving into an integrated, audit-ready system where speed, defensibility, and resilience determine winners
Seafood microbiological detection is moving decisively toward faster, more integrated, and more defensible programs as global supply chains grow more complex and expectations for proof of control rise. The most effective strategies recognize that testing is not merely a compliance step; it is a management tool that validates preventive controls, guides operational decisions, and protects customer trust.Transformative shifts-particularly rapid molecular adoption, expanded environmental monitoring, and digital traceability-are reshaping how organizations balance speed with credibility. At the same time, policy and trade dynamics such as prospective 2025 tariff pressures can amplify the need for supplier agility, matrix-fit validation, and standardized governance across sites.
Segmentation and regional perspectives underscore that there is no one-size-fits-all solution. The right program depends on product form, target organisms, decision timelines, and the maturity of local infrastructure and buyer requirements. Organizations that design testing architectures around these realities, invest in capability building, and embed results into disciplined corrective-action systems will be best positioned to reduce risk while sustaining throughput.
Ultimately, the leaders in this space will be those who treat microbiological detection as an adaptable system-one that can absorb supply shifts, support investigations, and provide audit-ready evidence-without slowing the business. This mindset turns microbiology from a cost center into a source of operational resilience and brand protection.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Seafood Microbiological Detection Market
Companies Mentioned
The key companies profiled in this Seafood Microbiological Detection market report include:- 3M Company
- ALS Limited
- Bio-Rad Laboratories, Inc.
- bioMérieux SA
- Envirocare Labs Private Limited
- Eurofins Scientific SE
- FOSS A/S
- Intertek Group plc
- Intertek India Private Limited
- Microbac Laboratories, Inc.
- Mérieux NutriSciences Corporation
- Neogen Corporation
- Pacific Rim Laboratories Inc.
- R-Biopharm AG
- Revvity, Inc.
- Romer Labs Division Holding GmbH
- SGS SA
- Shimadzu Corporation
- Thermo Fisher Scientific Inc.
- TÜV SÜD AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
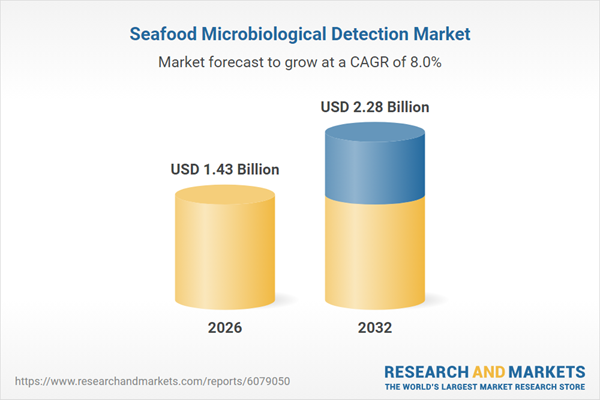
| Estimated Market Value ( USD | $ 1.43 Billion |
| Forecasted Market Value ( USD | $ 2.28 Billion |
| Compound Annual Growth Rate | 7.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |