Speak directly to the analyst to clarify any post sales queries you may have.
Budesonide & Formoterol inhalation stands at the center of modern respiratory care as competition, access, and device expectations intensify
Budesonide & Formoterol inhalation remains one of the most clinically established and commercially important fixed-dose combinations in inhaled respiratory therapy, bringing together an inhaled corticosteroid to reduce airway inflammation and a long-acting beta-agonist to maintain bronchodilation. Its role spans both asthma and chronic obstructive pulmonary disease (COPD) care pathways, where long-term control, reduction of exacerbations, and symptom stability are priorities for clinicians and payers alike. As treatment paradigms evolve, the combination continues to sit at the intersection of guideline-driven medicine, patient adherence realities, and the practical constraints of device technique.In recent years, the category has been reshaped by intensifying generic competition, expanding expectations for real-world outcomes, and heightened attention to inhaler usability and environmental considerations. At the same time, policy pressures and procurement scrutiny have increased the need for defensible value narratives that extend beyond clinical efficacy to include total cost of care and measurable adherence improvements. For manufacturers and stakeholders across the supply chain, Budesonide & Formoterol inhalation is therefore not merely a mature therapeutic area; it is a proving ground for how respiratory brands and generics compete on differentiation when molecules are well understood and access dynamics are increasingly complex.
This executive summary frames the strategic landscape through the most material forces shaping adoption and profitability. It highlights the market’s structural shifts, the implications of new tariff conditions in the United States, the segmentation patterns that define how products are selected and used, and the regional and company-level considerations that guide investment, partnering, and go-to-market design.
Competition is shifting from molecule differentiation to execution excellence as adherence, device fit, and procurement rules reshape demand
The competitive landscape is undergoing a shift from molecule-centric differentiation to execution-centric advantage, where supply reliability, contracting sophistication, and device familiarity can outweigh marginal formulation distinctions. As more alternatives enter the market, stakeholders are rebalancing decisions toward continuity of therapy, patient training burden, and switching friction within formularies and health systems. This has made commercial success increasingly dependent on operational excellence and customer engagement rather than on clinical awareness alone.Another transformative change is the growing emphasis on adherence and inhaler technique as determinants of outcomes. Clinicians and integrated delivery networks are placing renewed focus on whether the chosen inhaler format aligns with patient capability, comorbidities, and dexterity limitations. Consequently, device education, pharmacist intervention, and digital adherence supports-when available-are moving from “nice-to-have” services to core components of a differentiated offering. In parallel, the use of real-world evidence is expanding to validate persistence and exacerbation reduction in routine practice, elevating the importance of data partnerships and outcomes measurement.
Regulatory and procurement dynamics are also reshaping the category. As health systems sharpen therapeutic interchange policies and substitution pathways, manufacturers must anticipate switching events and ensure that messaging supports safe transitions and consistent technique. Meanwhile, pressure to reduce healthcare costs is reinforcing preference for cost-effective options, yet that preference is increasingly tested by supply volatility and the hidden costs associated with poor adherence or device misuse.
Finally, sustainability expectations are beginning to influence inhaler conversations in respiratory care, even where Budesonide & Formoterol products are not uniformly associated with the highest environmental impact categories. Institutions and national health programs are exploring procurement policies that integrate environmental criteria, and manufacturers are responding with packaging, logistics, and lifecycle initiatives. Over time, the ability to document and improve environmental performance may become a supportive differentiator in tender processes and institutional contracting, especially in regions where “green procurement” is advancing from policy intent to measurable requirements.
United States tariffs in 2025 are poised to reshape inhalation supply economics, forcing new sourcing, pricing, and continuity strategies
United States tariff actions anticipated for 2025 introduce a material layer of cost and planning complexity for Budesonide & Formoterol inhalation stakeholders, particularly where active pharmaceutical ingredients, excipients, device components, packaging materials, or finished-dose products touch cross-border supply routes. Even when tariffs do not target a specific medicine directly, they can raise landed costs through upstream inputs such as plastics, aluminum components, electronics for manufacturing lines, or specialty films used in packaging and labeling. The result is that procurement teams and manufacturers must treat tariff exposure as a system-wide risk rather than a narrow trade compliance issue.For manufacturers, the immediate impact is margin pressure and increased variability in cost of goods, especially for price-sensitive channels where passing through costs is constrained by contracting terms or reimbursement ceilings. This can intensify the need for dual sourcing, inventory buffering, and renegotiation of supplier agreements that previously assumed stable import economics. Over time, the tariff environment may also affect decisions about where to place final assembly for inhaler devices, where to qualify secondary packaging operations, and how to structure intercompany transfer pricing to remain compliant while preserving competitiveness.
The downstream effects extend to wholesalers, pharmacies, payers, and providers. If tariffs contribute to intermittent supply tightness for select presentations, formularies may become more conservative about non-medical switching, while health systems could increase preference for suppliers with robust continuity plans. In parallel, competitive bidding and contracting may incorporate stronger service-level expectations, such as fill-rate guarantees, rapid backorder resolution, and transparent notification protocols.
Strategically, tariffs may accelerate a re-localization mindset for critical inputs or, at minimum, a “near-shoring” approach that reduces exposure to high-volatility trade corridors. However, moving supply chains is rarely frictionless in inhalation products due to device-specific tooling, validation requirements, and regulatory filings. Therefore, the most durable response is a portfolio of mitigations: targeted component redesign to reduce tariffed inputs, diversification of qualified suppliers, and scenario-based pricing strategies that define how costs are absorbed, shared, or offset through manufacturing productivity. In 2025 and beyond, the companies that operationalize these levers fastest are likely to face fewer disruptions and maintain stronger negotiating positions across commercial channels.
Segmentation reveals how brand status, device format, indication, channel dynamics, and care settings jointly determine adoption patterns
Segmentation patterns in Budesonide & Formoterol inhalation reflect how clinical needs, device capabilities, and purchasing structures converge in real-world care. When viewed by product type, the balance between branded and generic offerings continues to shape contracting leverage and substitution behavior, with stakeholders weighing acquisition cost against confidence in supply reliability, device familiarity, and support services that reduce technique errors. This dynamic tends to intensify in environments where therapeutic interchange is actively managed, making the value proposition as much about implementation as about the label.Looking through the lens of dosage form and device platform, inhaler design and usability meaningfully influence adherence and patient satisfaction. Differences between metered-dose inhalers and dry powder inhalers can translate into distinct training needs, inspiratory flow requirements, and perceptions of convenience. In practice, this means that segment demand can shift based on population characteristics such as age, comorbidities affecting coordination, or the prevalence of severe disease where technique reliability is critical. These factors also shape how providers and pharmacists prioritize education and follow-up, especially after initiation or a switch.
By application, asthma and COPD drivers diverge in ways that influence prescribing intensity, regimen stability, and the role of step-up or step-down therapy. Asthma segments are increasingly influenced by guideline evolution, patient preference for simplified regimens, and the clinical emphasis on reducing exacerbation risk. COPD segments, meanwhile, are shaped by symptom burden, exacerbation history, and the broader shift toward individualized inhaled therapy choices that balance bronchodilation and anti-inflammatory strategies. Across both applications, comorbidity profiles and smoking history can affect treatment persistence and the likelihood of escalation to triple therapy, which in turn influences the duration a patient remains within the Budesonide & Formoterol segment.
Distribution channel segmentation adds another layer of differentiation because purchasing behavior and switching friction vary by setting. Hospital pharmacies emphasize formulary alignment, continuity of supply, and standardized device types that reduce inpatient training variation. Retail pharmacies are often the practical point of adherence intervention, where substitution, patient counseling, and refill behavior influence outcomes. Online pharmacies are gaining relevance where policy and reimbursement allow, and they can increase convenience and refill consistency, although they also require strong patient support to ensure technique remains correct.
Finally, end-user segmentation underscores how the same therapy behaves differently depending on care delivery context. Hospitals focus on acute stabilization and discharge planning, which can make inhaler selection sensitive to transitions of care. Clinics and specialty practices concentrate on chronic management and monitoring, where titration and patient education are central. Homecare settings, including patients managing complex regimens, place the premium on ease of use, caregiver involvement, and the availability of ongoing support. Understanding these segmentation dynamics is essential because competitive advantage increasingly comes from aligning product, device, education, and access strategy to the realities of each care pathway.
Regional demand is shaped by procurement systems, reimbursement rules, and localized care delivery models that change how therapy is chosen
Regional dynamics in Budesonide & Formoterol inhalation are shaped by differences in reimbursement models, prescribing guidelines, tendering practices, and the maturity of generic competition. In the Americas, payer-driven formulary management and contracting discipline strongly influence product selection, and stakeholders increasingly prioritize suppliers that can sustain consistent availability while supporting adherence and patient education. The region’s clinical infrastructure supports broad use across asthma and COPD, yet access decisions can vary widely depending on insurance design and the negotiating power of large health systems.Across Europe, Middle East & Africa, public procurement and tender mechanisms frequently set the tone for utilization, and switching can be more centralized when health authorities update preferred products. This can create rapid shifts in volume toward suppliers that win tenders, while also raising the importance of device familiarity programs to reduce errors following transitions. At the same time, variation within the region is pronounced: some markets emphasize cost containment above all, whereas others place greater weight on continuity plans, environmental criteria, and real-world outcomes documentation.
In Asia-Pacific, growth in diagnosis, expanding access to chronic respiratory management, and increasing urban pollution burdens in certain geographies contribute to sustained demand. However, the region’s diversity in healthcare financing and distribution infrastructure means that uptake is uneven, with some countries favoring hospital-centric procurement and others seeing stronger retail pharmacy influence. Manufacturers that tailor device training, language-specific education materials, and channel strategies to local practice patterns tend to outperform those that rely on a one-size-fits-all commercial model.
Taken together, regional insights reinforce a central theme: success depends on aligning the offer to how care is financed and delivered in each geography. As cross-border supply conditions and policy expectations evolve, region-specific resilience-spanning regulatory readiness, localized packaging or assembly where feasible, and partnerships that support patient use-becomes a decisive capability rather than a secondary consideration.
Leading players differentiate through device reliability, compliant scale-up, market access execution, and resilience across complex supply networks
Key companies competing in Budesonide & Formoterol inhalation are increasingly defined by how well they orchestrate manufacturing scale, device know-how, regulatory execution, and commercial access. Originator and established respiratory players retain advantages in clinical trust, device recognition, and long-standing relationships with prescribers and health systems. These strengths can translate into resilience during periods of supply instability or policy-driven switching, especially when stakeholders perceive lower risk in continuity and patient technique.At the same time, generic and value-focused manufacturers have sharpened their competitive playbooks. Success is tied to efficient cost structures, fast and compliant market entry, and the ability to meet stringent quality expectations for complex combination products. In inhalation, the device component raises the bar: engineering precision, reproducibility, and human factors considerations can become as important as the formulation itself. As a result, companies that invest in device validation capabilities and robust post-market quality systems often gain credibility with institutional buyers.
Partnerships and specialized suppliers also influence the competitive map. Inhaler components, tooling, and packaging ecosystems can create dependencies that reward companies with diversified vendor networks and strong technical transfer processes. Moreover, organizations that can support education-through patient materials, pharmacy training, or field-based clinical support-are better positioned to protect persistence after a formulary change or substitution event.
Overall, competitive differentiation is moving toward a blended model: reliable and compliant supply, credible device performance, and market access excellence. Companies that treat these elements as integrated-rather than siloed into manufacturing, regulatory, and sales functions-are more likely to sustain adoption in a category where price pressure is real but switching costs and outcomes accountability are rising.
Actionable moves for leaders center on resilient sourcing, adherence-driven differentiation, evidence aligned to payer needs, and policy-ready execution
Industry leaders should prioritize supply chain resilience as a commercial strategy, not only as an operational safeguard. This means mapping tariff exposure across APIs, device subcomponents, and packaging inputs; qualifying alternates that can meet inhalation-grade specifications; and establishing clear continuity commitments for customers. In parallel, leaders should design contracting approaches that incorporate service levels-such as fill-rate performance, substitution support, and transition materials-so buyers can evaluate the total risk-adjusted value rather than unit price alone.Next, companies should strengthen adherence and technique support because it directly influences persistence, exacerbations, and stakeholder confidence. Investments in clear instructions, multilingual training content, pharmacist enablement, and practical patient support tools can reduce misuse and protect outcomes, particularly after switching events. Where appropriate, leaders can collaborate with providers and pharmacies to standardize technique checks and embed them in routine care, positioning the product as part of an outcomes-oriented pathway.
Leaders should also align evidence generation with real-world decision criteria. Rather than relying solely on clinical trial familiarity, organizations can develop real-world datasets that speak to persistence, refill behavior, and exacerbation-related utilization, framed in ways that resonate with payers and integrated delivery networks. This approach is especially relevant in environments where tender decisions or formulary status can shift quickly and where decision-makers seek reassurance that transitions will not increase downstream costs.
Finally, portfolio and lifecycle strategy should anticipate evolving policy expectations, including sustainability considerations and procurement transparency. Even incremental progress in packaging optimization, logistics efficiency, and lifecycle documentation can support institutional discussions. When combined with disciplined regulatory planning for manufacturing changes, this can help companies adapt without triggering avoidable disruptions. The most effective leaders will treat these moves as a coordinated roadmap-linking operations, access, medical, and commercial teams around a common objective: sustained patient outcomes at predictable cost and dependable availability.
A triangulated methodology combines regulatory review, stakeholder interviews, and cross-validation to produce decision-ready market intelligence
This research methodology integrates primary and secondary research to build a cohesive view of the Budesonide & Formoterol inhalation landscape without relying on a single lens. Secondary research draws from publicly available regulatory documents, product labeling and approvals, procurement and tender frameworks, policy publications, and corporate disclosures. This foundation is used to map the competitive environment, identify evolving requirements for inhalation products, and understand how access and distribution models differ by region.Primary research complements this base through structured discussions with knowledgeable stakeholders across the value chain, including participants with experience in respiratory prescribing, pharmacy operations, payer and formulary decision-making, distribution logistics, and manufacturing or regulatory affairs. These conversations are used to validate assumptions, clarify real-world switching practices, and surface practical constraints that may not be explicit in formal documentation.
The analysis uses triangulation to reconcile divergent inputs and reduce bias. Insights are cross-checked across multiple perspectives, and emphasis is placed on repeatable patterns such as consistent procurement behaviors, recurring supply bottlenecks, or common adherence challenges. The resulting synthesis focuses on strategic implications, segment behavior, and actionable considerations for stakeholders evaluating product positioning, partnering, or operational investment.
Quality control measures include internal consistency checks, terminology standardization across regions and channels, and a deliberate separation of observed market behaviors from interpretive conclusions. This approach supports decision-ready insights that remain grounded in verifiable information and expert validation.
The category’s future favors integrated players that align device strategy, access execution, and resilient supply with real-world care pathways
Budesonide & Formoterol inhalation continues to play a pivotal role in chronic respiratory disease management, but the basis of competition is evolving. Stakeholders are no longer choosing products solely on clinical familiarity; they are weighing device usability, adherence support, supply continuity, and the operational burden of switching. This reality elevates the importance of execution-especially in environments where procurement is centralized and where substitution can occur at scale.As 2025 tariff conditions reshape cost structures and sourcing priorities in the United States, operational resilience becomes inseparable from commercial performance. Companies that anticipate cost volatility, diversify critical inputs, and communicate continuity plans credibly will be better positioned to sustain access and trust. At the same time, region-specific procurement norms and distribution models demand tailored strategies rather than global uniformity.
Ultimately, the organizations that win in this category will be those that integrate device strategy, evidence planning, access excellence, and supply discipline into one coherent operating model. By aligning these capabilities to the segmentation realities of indications, channels, and care settings, industry leaders can protect outcomes while navigating intensifying price and policy pressure.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Budesonide & Formoterol Inhalation Market
Companies Mentioned
The key companies profiled in this Budesonide & Formoterol Inhalation market report include:- Almirall, S.A.
- Amneal Pharmaceuticals, Inc.
- Apotex Inc.
- AstraZeneca PLC
- Chiesi Farmaceutici S.p.A.
- Cipla Europe NV
- Dr. Reddy's Laboratories Limited
- Glenmark Pharmaceuticals Limited
- Hetero Drugs Limited
- Hikma Pharmaceuticals PLC
- Intas Pharmaceuticals Limited
- Lupin Limited
- Macleods Pharmaceuticals Ltd.
- MSN Laboratories Private Limited
- Mundipharma International Limited
- Orion Corporation
- Perrigo Company plc
- Sandoz International GmbH
- Sun Pharmaceutical Industries Limited
- Teva Pharmaceutical Industries Limited
- Torrent Pharmaceuticals Ltd.
- Viatris Inc.
- Zydus Lifesciences Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
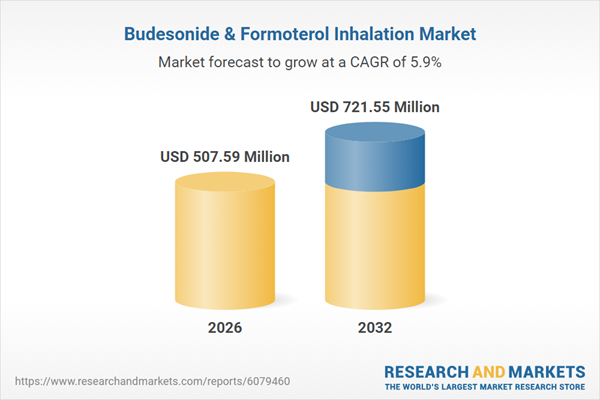
| Estimated Market Value ( USD | $ 507.59 Million |
| Forecasted Market Value ( USD | $ 721.55 Million |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |