1h Free Analyst Time
Speak directly to the analyst to clarify any post sales queries you may have.
The biopharmaceutical-grade TPE tubing market is experiencing robust expansion as the sector shifts toward advanced materials and supply chain strategies to ensure compliance, process integrity, and reliable operational performance.
Market Snapshot: Biopharmaceutical Grade TPE Tubing Market
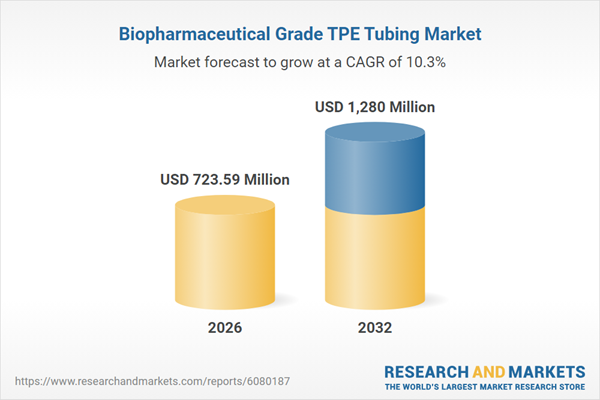
The market for biopharmaceutical-grade TPE tubing is projected to rise from USD 645.83 million in 2025 to USD 723.59 million in 2026, advancing at a CAGR of 10.27% and expected to reach USD 1.28 billion by 2032. This momentum is driven by escalating demand for sterile single-use systems, process intensification in drug manufacturing, and heightened global quality standards.
Scope & Segmentation
- Application Types: Includes chromatography, filtration, cell culture, fluid transfer, pump cycling, connector interfaces, and single-use assemblies.
- Material Grades: Segmented into high, medium, and soft-hardness thermoplastic elastomer formulations tailored for mechanical robustness, flexibility, and biocompatibility.
- Manufacturing Processes: Covers extrusion, co-extrusion, two-layer and three-layer constructions for balanced inner surface chemistry and external handling strength.
- Sterilization Methods: Encompasses autoclave, ethylene oxide, E-beam, and gamma techniques validated for compatibility and regulatory compliance.
- Distribution Channels: Spans aftermarket distribution, direct sales, OEM partnerships, and online commerce for tailored supply solutions.
- Geographic Regions: Analysis spans Americas, Europe, Middle East & Africa, and Asia-Pacific, examining regional procurement dynamics, standards, and supply chain resilience.
Key Takeaways
- Technical evaluation of TPE tubing now necessitates multidisciplinary input, focusing on chemical resistance, biocompatibility, durability, and validated sterilization performance.
- Adoption of single-use systems is accelerating, increasing requirements for tubing that seamlessly integrates with assemblies, maintains sterility, and supports quick product changeovers.
- Co-extrusion and multi-layer manufacturing enable differentiated products that balance interior biocompatibility with exterior mechanical resilience, optimizing tubing for diverse workflows.
- Sustainability and supply chain transparency are increasingly influencing supplier selection, prompting buyers to seek local or regional sources with robust quality documentation and rapid logistics.
- Regional regulatory expectations drive suppliers to customize engagement, documentation, and inventory models to fit the operational tempos and compliance requirements of each hub.
Tariff Impact on Sourcing and Supplier Qualification
Recent US tariff changes have altered the supply landscape for specialty TPE tubing. Organizations are adopting multi-source strategies, intensifying supplier qualification, and scrutinizing raw material origins to manage landed costs, minimize customs risks, and maintain regulatory traceability. Many are exploring regional manufacturing partnerships and early integration of procurement teams in specification development to reinforce supply continuity and mitigate the impact of changing trade policies.
Methodology & Data Sources
This report utilizes a mixed-method approach, combining direct interviews with industry stakeholders—including process engineers and procurement professionals—with detailed technical validation and secondary research into global standards and supplier documentation. Data triangulation was used to corroborate supplier claims and to benchmark product performance across key use cases.
Why This Report Matters
- Provides in-depth insights into the evolving criteria for material selection, regulatory alignment, and operational risk mitigation for procurement leaders and technical teams.
- Enables benchmarking of supplier capabilities and strategic evaluation of sourcing models amid shifting trade, compliance, and sustainability environments.
Conclusion
Biopharmaceutical-grade TPE tubing is transitioning from a commodity item to a strategically validated component within bioprocess supply chains. Suppliers offering robust materials expertise, compliance-ready documentation, and flexible regional logistics will be positioned for long-term growth as industry standards and technical expectations continue to rise.
Table of Contents
1. Preface
1.1. Objectives of the Study
1.2. Market Segmentation & Coverage
1.3. Years Considered for the Study
1.4. Currency & Pricing
1.5. Language
1.6. Stakeholders
1.2. Market Segmentation & Coverage
1.3. Years Considered for the Study
1.4. Currency & Pricing
1.5. Language
1.6. Stakeholders
2. Research Methodology
2.1. Define: Research Objective
2.2. Determine: Research Design
2.3. Prepare: Research Instrument
2.4. Collect: Data Source
2.5. Analyze: Data Interpretation
2.6. Formulate: Data Verification
2.7. Publish: Research Report
2.8. Repeat: Report Update
2.2. Determine: Research Design
2.3. Prepare: Research Instrument
2.4. Collect: Data Source
2.5. Analyze: Data Interpretation
2.6. Formulate: Data Verification
2.7. Publish: Research Report
2.8. Repeat: Report Update
4. Market Overview
4.1. Introduction
4.2. Market Sizing & Forecasting
4.2. Market Sizing & Forecasting
5. Market Dynamics
5.1. Rise in demand for single-use TPE tubing in biologics production due to contamination reduction
5.2. Increasing adoption of gamma-stable low extractables TPE tubing in cell and gene therapy manufacturing
5.3. Shift towards custom-engineered TPE tubing with advanced sterilization compatibility for continuous bioprocessing
5.4. Integration of TPE tubing in closed system single-use bioreactors to enhance aseptic conditions
5.5. Development of eco-friendly recyclable TPE materials in biopharmaceutical tubing to meet sustainability targets
5.6. Intensified regulatory scrutiny on extractables and leachables testing for TPE tubing in therapeutic drug manufacturing
5.7. Formation of strategic partnerships between TPE tubing suppliers and biopharma firms to co-develop application specific solutions
5.8. Technological advancements in coextrusion multilayer TPE tubing to optimize flow characteristics and minimize adsorption
5.2. Increasing adoption of gamma-stable low extractables TPE tubing in cell and gene therapy manufacturing
5.3. Shift towards custom-engineered TPE tubing with advanced sterilization compatibility for continuous bioprocessing
5.4. Integration of TPE tubing in closed system single-use bioreactors to enhance aseptic conditions
5.5. Development of eco-friendly recyclable TPE materials in biopharmaceutical tubing to meet sustainability targets
5.6. Intensified regulatory scrutiny on extractables and leachables testing for TPE tubing in therapeutic drug manufacturing
5.7. Formation of strategic partnerships between TPE tubing suppliers and biopharma firms to co-develop application specific solutions
5.8. Technological advancements in coextrusion multilayer TPE tubing to optimize flow characteristics and minimize adsorption
6. Market Insights
6.1. Porter’s Five Forces Analysis
6.2. PESTLE Analysis
6.2. PESTLE Analysis
8. Biopharmaceutical Grade TPE Tubing Market, by Application
8.1. Introduction
8.2. Cell Culture
8.3. Chromatography
8.3.1. HPLC Tubing
8.3.2. Ion Exchange Tubing
8.4. Filtration
8.4.1. Depth Filtration
8.4.2. Membrane Filtration
8.5. Fluid Transfer
8.5.1. Connector Tubing
8.5.2. Pump Tubing
8.6. Single Use System
8.6.1. Assemblies
8.6.2. Bags
8.2. Cell Culture
8.3. Chromatography
8.3.1. HPLC Tubing
8.3.2. Ion Exchange Tubing
8.4. Filtration
8.4.1. Depth Filtration
8.4.2. Membrane Filtration
8.5. Fluid Transfer
8.5.1. Connector Tubing
8.5.2. Pump Tubing
8.6. Single Use System
8.6.1. Assemblies
8.6.2. Bags
9. Biopharmaceutical Grade TPE Tubing Market, by Material Grade
9.1. Introduction
9.2. High Hardness
9.3. Medium Hardness
9.4. Soft Hardness
9.2. High Hardness
9.3. Medium Hardness
9.4. Soft Hardness
10. Biopharmaceutical Grade TPE Tubing Market, by Manufacturing Process
10.1. Introduction
10.2. Co-Extrusion
10.2.1. Three Layer
10.2.2. Two Layer
10.3. Extrusion
10.2. Co-Extrusion
10.2.1. Three Layer
10.2.2. Two Layer
10.3. Extrusion
11. Biopharmaceutical Grade TPE Tubing Market, by Sterilization Method
11.1. Introduction
11.2. Autoclave
11.3. E Beam
11.4. Ethylene Oxide
11.5. Gamma
11.2. Autoclave
11.3. E Beam
11.4. Ethylene Oxide
11.5. Gamma
12. Biopharmaceutical Grade TPE Tubing Market, by Distribution Channel
12.1. Introduction
12.2. Aftermarket Distribution
12.3. Direct Sales
12.4. Oem
12.5. Online Distribution
12.2. Aftermarket Distribution
12.3. Direct Sales
12.4. Oem
12.5. Online Distribution
13. Americas Biopharmaceutical Grade TPE Tubing Market
13.1. Introduction
13.2. United States
13.3. Canada
13.4. Mexico
13.5. Brazil
13.6. Argentina
13.2. United States
13.3. Canada
13.4. Mexico
13.5. Brazil
13.6. Argentina
14. Europe, Middle East & Africa Biopharmaceutical Grade TPE Tubing Market
14.1. Introduction
14.2. United Kingdom
14.3. Germany
14.4. France
14.5. Russia
14.6. Italy
14.7. Spain
14.8. United Arab Emirates
14.9. Saudi Arabia
14.10. South Africa
14.11. Denmark
14.12. Netherlands
14.13. Qatar
14.14. Finland
14.15. Sweden
14.16. Nigeria
14.17. Egypt
14.18. Turkey
14.19. Israel
14.20. Norway
14.21. Poland
14.22. Switzerland
14.2. United Kingdom
14.3. Germany
14.4. France
14.5. Russia
14.6. Italy
14.7. Spain
14.8. United Arab Emirates
14.9. Saudi Arabia
14.10. South Africa
14.11. Denmark
14.12. Netherlands
14.13. Qatar
14.14. Finland
14.15. Sweden
14.16. Nigeria
14.17. Egypt
14.18. Turkey
14.19. Israel
14.20. Norway
14.21. Poland
14.22. Switzerland
15. Asia-Pacific Biopharmaceutical Grade TPE Tubing Market
15.1. Introduction
15.2. China
15.3. India
15.4. Japan
15.5. Australia
15.6. South Korea
15.7. Indonesia
15.8. Thailand
15.9. Philippines
15.10. Malaysia
15.11. Singapore
15.12. Vietnam
15.13. Taiwan
15.2. China
15.3. India
15.4. Japan
15.5. Australia
15.6. South Korea
15.7. Indonesia
15.8. Thailand
15.9. Philippines
15.10. Malaysia
15.11. Singapore
15.12. Vietnam
15.13. Taiwan
16. Competitive Landscape
16.1. Market Share Analysis, 2024
16.2. FPNV Positioning Matrix, 2024
16.3. Competitive Analysis
16.3.1. Compagnie de Saint-Gobain SA
16.3.2. Parker-Hannifin Corporation
16.3.3. Trelleborg AB (publ)
16.3.4. Freudenberg SE
16.3.5. Tekni-Plex, Inc.
16.3.6. Nordson Corporation
16.3.7. Spirax-Sarco Engineering plc
16.3.8. Avantor, Inc.
16.3.9. Zeus Industrial Products, Inc.
16.3.10. Teknor Apex Company
16.2. FPNV Positioning Matrix, 2024
16.3. Competitive Analysis
16.3.1. Compagnie de Saint-Gobain SA
16.3.2. Parker-Hannifin Corporation
16.3.3. Trelleborg AB (publ)
16.3.4. Freudenberg SE
16.3.5. Tekni-Plex, Inc.
16.3.6. Nordson Corporation
16.3.7. Spirax-Sarco Engineering plc
16.3.8. Avantor, Inc.
16.3.9. Zeus Industrial Products, Inc.
16.3.10. Teknor Apex Company
18. ResearchStatistics
19. ResearchContacts
20. ResearchArticles
21. Appendix
List of Figures
FIGURE 1. BIOPHARMACEUTICAL GRADE TPE TUBING MARKET RESEARCH PROCESS
FIGURE 2. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, 2018-2030 (USD MILLION)
FIGURE 3. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY REGION, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 4. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 5. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2024 VS 2030 (%)
FIGURE 6. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 7. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2024 VS 2030 (%)
FIGURE 8. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 9. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2024 VS 2030 (%)
FIGURE 10. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 11. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2024 VS 2030 (%)
FIGURE 12. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 13. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2024 VS 2030 (%)
FIGURE 14. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 15. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2024 VS 2030 (%)
FIGURE 16. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 17. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STATE, 2024 VS 2030 (%)
FIGURE 18. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STATE, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 19. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2024 VS 2030 (%)
FIGURE 20. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 21. ASIA-PACIFIC BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2024 VS 2030 (%)
FIGURE 22. ASIA-PACIFIC BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 23. BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SHARE, BY KEY PLAYER, 2024
FIGURE 24. BIOPHARMACEUTICAL GRADE TPE TUBING MARKET, FPNV POSITIONING MATRIX, 2024
FIGURE 25. BIOPHARMACEUTICAL GRADE TPE TUBING MARKET: RESEARCHAI
FIGURE 26. BIOPHARMACEUTICAL GRADE TPE TUBING MARKET: RESEARCHSTATISTICS
FIGURE 27. BIOPHARMACEUTICAL GRADE TPE TUBING MARKET: RESEARCHCONTACTS
FIGURE 28. BIOPHARMACEUTICAL GRADE TPE TUBING MARKET: RESEARCHARTICLES
FIGURE 2. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, 2018-2030 (USD MILLION)
FIGURE 3. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY REGION, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 4. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 5. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2024 VS 2030 (%)
FIGURE 6. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 7. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2024 VS 2030 (%)
FIGURE 8. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 9. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2024 VS 2030 (%)
FIGURE 10. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 11. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2024 VS 2030 (%)
FIGURE 12. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 13. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2024 VS 2030 (%)
FIGURE 14. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 15. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2024 VS 2030 (%)
FIGURE 16. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 17. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STATE, 2024 VS 2030 (%)
FIGURE 18. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STATE, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 19. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2024 VS 2030 (%)
FIGURE 20. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 21. ASIA-PACIFIC BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2024 VS 2030 (%)
FIGURE 22. ASIA-PACIFIC BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2024 VS 2025 VS 2030 (USD MILLION)
FIGURE 23. BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SHARE, BY KEY PLAYER, 2024
FIGURE 24. BIOPHARMACEUTICAL GRADE TPE TUBING MARKET, FPNV POSITIONING MATRIX, 2024
FIGURE 25. BIOPHARMACEUTICAL GRADE TPE TUBING MARKET: RESEARCHAI
FIGURE 26. BIOPHARMACEUTICAL GRADE TPE TUBING MARKET: RESEARCHSTATISTICS
FIGURE 27. BIOPHARMACEUTICAL GRADE TPE TUBING MARKET: RESEARCHCONTACTS
FIGURE 28. BIOPHARMACEUTICAL GRADE TPE TUBING MARKET: RESEARCHARTICLES
List of Tables
TABLE 1. BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SEGMENTATION & COVERAGE
TABLE 2. UNITED STATES DOLLAR EXCHANGE RATE, 2018-2024
TABLE 3. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, 2018-2024 (USD MILLION)
TABLE 4. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, 2025-2030 (USD MILLION)
TABLE 5. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY REGION, 2018-2024 (USD MILLION)
TABLE 6. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY REGION, 2025-2030 (USD MILLION)
TABLE 7. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2018-2024 (USD MILLION)
TABLE 8. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2025-2030 (USD MILLION)
TABLE 9. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 10. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 11. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CELL CULTURE, BY REGION, 2018-2024 (USD MILLION)
TABLE 12. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CELL CULTURE, BY REGION, 2025-2030 (USD MILLION)
TABLE 13. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, BY REGION, 2018-2024 (USD MILLION)
TABLE 14. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, BY REGION, 2025-2030 (USD MILLION)
TABLE 15. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY HPLC TUBING, BY REGION, 2018-2024 (USD MILLION)
TABLE 16. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY HPLC TUBING, BY REGION, 2025-2030 (USD MILLION)
TABLE 17. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ION EXCHANGE TUBING, BY REGION, 2018-2024 (USD MILLION)
TABLE 18. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ION EXCHANGE TUBING, BY REGION, 2025-2030 (USD MILLION)
TABLE 19. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 20. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 21. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, BY REGION, 2018-2024 (USD MILLION)
TABLE 22. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, BY REGION, 2025-2030 (USD MILLION)
TABLE 23. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DEPTH FILTRATION, BY REGION, 2018-2024 (USD MILLION)
TABLE 24. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DEPTH FILTRATION, BY REGION, 2025-2030 (USD MILLION)
TABLE 25. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MEMBRANE FILTRATION, BY REGION, 2018-2024 (USD MILLION)
TABLE 26. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MEMBRANE FILTRATION, BY REGION, 2025-2030 (USD MILLION)
TABLE 27. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 28. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 29. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, BY REGION, 2018-2024 (USD MILLION)
TABLE 30. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, BY REGION, 2025-2030 (USD MILLION)
TABLE 31. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CONNECTOR TUBING, BY REGION, 2018-2024 (USD MILLION)
TABLE 32. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CONNECTOR TUBING, BY REGION, 2025-2030 (USD MILLION)
TABLE 33. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY PUMP TUBING, BY REGION, 2018-2024 (USD MILLION)
TABLE 34. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY PUMP TUBING, BY REGION, 2025-2030 (USD MILLION)
TABLE 35. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 36. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 37. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, BY REGION, 2018-2024 (USD MILLION)
TABLE 38. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, BY REGION, 2025-2030 (USD MILLION)
TABLE 39. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ASSEMBLIES, BY REGION, 2018-2024 (USD MILLION)
TABLE 40. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ASSEMBLIES, BY REGION, 2025-2030 (USD MILLION)
TABLE 41. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY BAGS, BY REGION, 2018-2024 (USD MILLION)
TABLE 42. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY BAGS, BY REGION, 2025-2030 (USD MILLION)
TABLE 43. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 44. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 45. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 46. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 47. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY HIGH HARDNESS, BY REGION, 2018-2024 (USD MILLION)
TABLE 48. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY HIGH HARDNESS, BY REGION, 2025-2030 (USD MILLION)
TABLE 49. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MEDIUM HARDNESS, BY REGION, 2018-2024 (USD MILLION)
TABLE 50. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MEDIUM HARDNESS, BY REGION, 2025-2030 (USD MILLION)
TABLE 51. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SOFT HARDNESS, BY REGION, 2018-2024 (USD MILLION)
TABLE 52. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SOFT HARDNESS, BY REGION, 2025-2030 (USD MILLION)
TABLE 53. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 54. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 55. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, BY REGION, 2018-2024 (USD MILLION)
TABLE 56. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, BY REGION, 2025-2030 (USD MILLION)
TABLE 57. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY THREE LAYER, BY REGION, 2018-2024 (USD MILLION)
TABLE 58. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY THREE LAYER, BY REGION, 2025-2030 (USD MILLION)
TABLE 59. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY TWO LAYER, BY REGION, 2018-2024 (USD MILLION)
TABLE 60. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY TWO LAYER, BY REGION, 2025-2030 (USD MILLION)
TABLE 61. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 62. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 63. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY EXTRUSION, BY REGION, 2018-2024 (USD MILLION)
TABLE 64. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY EXTRUSION, BY REGION, 2025-2030 (USD MILLION)
TABLE 65. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 66. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 67. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY AUTOCLAVE, BY REGION, 2018-2024 (USD MILLION)
TABLE 68. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY AUTOCLAVE, BY REGION, 2025-2030 (USD MILLION)
TABLE 69. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY E BEAM, BY REGION, 2018-2024 (USD MILLION)
TABLE 70. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY E BEAM, BY REGION, 2025-2030 (USD MILLION)
TABLE 71. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ETHYLENE OXIDE, BY REGION, 2018-2024 (USD MILLION)
TABLE 72. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ETHYLENE OXIDE, BY REGION, 2025-2030 (USD MILLION)
TABLE 73. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY GAMMA, BY REGION, 2018-2024 (USD MILLION)
TABLE 74. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY GAMMA, BY REGION, 2025-2030 (USD MILLION)
TABLE 75. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 76. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 77. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY AFTERMARKET DISTRIBUTION, BY REGION, 2018-2024 (USD MILLION)
TABLE 78. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY AFTERMARKET DISTRIBUTION, BY REGION, 2025-2030 (USD MILLION)
TABLE 79. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DIRECT SALES, BY REGION, 2018-2024 (USD MILLION)
TABLE 80. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DIRECT SALES, BY REGION, 2025-2030 (USD MILLION)
TABLE 81. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY OEM, BY REGION, 2018-2024 (USD MILLION)
TABLE 82. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY OEM, BY REGION, 2025-2030 (USD MILLION)
TABLE 83. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ONLINE DISTRIBUTION, BY REGION, 2018-2024 (USD MILLION)
TABLE 84. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ONLINE DISTRIBUTION, BY REGION, 2025-2030 (USD MILLION)
TABLE 85. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 86. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 87. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 88. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 89. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 90. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 91. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 92. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 93. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 94. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 95. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 96. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 97. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 98. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 99. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 100. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 101. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 102. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 103. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 104. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 105. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2018-2024 (USD MILLION)
TABLE 106. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2025-2030 (USD MILLION)
TABLE 107. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 108. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 109. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 110. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 111. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 112. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 113. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 114. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 115. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 116. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 117. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 118. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 119. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 120. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 121. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 122. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 123. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 124. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 125. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 126. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 127. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STATE, 2018-2024 (USD MILLION)
TABLE 128. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STATE, 2025-2030 (USD MILLION)
TABLE 129. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 130. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 131. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 132. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 133. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 134. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 135. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 136. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 137. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 138. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 139. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 140. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 141. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 142. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 143. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 144. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 145. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 146. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 147. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 148. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 149. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 150. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 151. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 152. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 153. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 154. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 155. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 156. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 157. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 158. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 159. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 160. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 161. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 162. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 163. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 164. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 165. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 166. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 167. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 168. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 169. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 170. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 171. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 172. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 173. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 174. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 175. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 176. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 177. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 178. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 179. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 180. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 181. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 182. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 183. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 184. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 185. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 186. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 187. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 188. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 189. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 190. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 191. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 192. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 193. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 194. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 195. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 196. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 197. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 198. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 199. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 200. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 201. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 202. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 203. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 204. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 205. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 206. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 207. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 208. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 209. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 210. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 211. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 212. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 213. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 214. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 215. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 216. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 217. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 218. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 219. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 220. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 221. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 222. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 223. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 224. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 225. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 226. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 227. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 228. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 229. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2018-2024 (USD MILLION)
TABLE 230. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2025-2030 (USD MILLION)
TABLE 231. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 232. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 233. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 234. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 235. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 236. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 237. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 238. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 239. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 240. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 241. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 242. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 243. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 244. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 245. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 246. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 247. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 248. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 249. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 250. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 251. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 252. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 253. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 254. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 255. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 256. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 257. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 258. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 259. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 260. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 261. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 262. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 263. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 264. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 265. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 266. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 267. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 268. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 269. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 270. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 271. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 272. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 273. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 274. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 275. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 276. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 277. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 278. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 279. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 280. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 281. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 282. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 283. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 284. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 285. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 286. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 287. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 288. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STE
TABLE 2. UNITED STATES DOLLAR EXCHANGE RATE, 2018-2024
TABLE 3. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, 2018-2024 (USD MILLION)
TABLE 4. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, 2025-2030 (USD MILLION)
TABLE 5. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY REGION, 2018-2024 (USD MILLION)
TABLE 6. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY REGION, 2025-2030 (USD MILLION)
TABLE 7. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2018-2024 (USD MILLION)
TABLE 8. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2025-2030 (USD MILLION)
TABLE 9. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 10. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 11. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CELL CULTURE, BY REGION, 2018-2024 (USD MILLION)
TABLE 12. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CELL CULTURE, BY REGION, 2025-2030 (USD MILLION)
TABLE 13. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, BY REGION, 2018-2024 (USD MILLION)
TABLE 14. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, BY REGION, 2025-2030 (USD MILLION)
TABLE 15. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY HPLC TUBING, BY REGION, 2018-2024 (USD MILLION)
TABLE 16. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY HPLC TUBING, BY REGION, 2025-2030 (USD MILLION)
TABLE 17. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ION EXCHANGE TUBING, BY REGION, 2018-2024 (USD MILLION)
TABLE 18. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ION EXCHANGE TUBING, BY REGION, 2025-2030 (USD MILLION)
TABLE 19. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 20. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 21. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, BY REGION, 2018-2024 (USD MILLION)
TABLE 22. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, BY REGION, 2025-2030 (USD MILLION)
TABLE 23. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DEPTH FILTRATION, BY REGION, 2018-2024 (USD MILLION)
TABLE 24. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DEPTH FILTRATION, BY REGION, 2025-2030 (USD MILLION)
TABLE 25. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MEMBRANE FILTRATION, BY REGION, 2018-2024 (USD MILLION)
TABLE 26. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MEMBRANE FILTRATION, BY REGION, 2025-2030 (USD MILLION)
TABLE 27. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 28. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 29. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, BY REGION, 2018-2024 (USD MILLION)
TABLE 30. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, BY REGION, 2025-2030 (USD MILLION)
TABLE 31. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CONNECTOR TUBING, BY REGION, 2018-2024 (USD MILLION)
TABLE 32. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CONNECTOR TUBING, BY REGION, 2025-2030 (USD MILLION)
TABLE 33. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY PUMP TUBING, BY REGION, 2018-2024 (USD MILLION)
TABLE 34. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY PUMP TUBING, BY REGION, 2025-2030 (USD MILLION)
TABLE 35. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 36. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 37. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, BY REGION, 2018-2024 (USD MILLION)
TABLE 38. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, BY REGION, 2025-2030 (USD MILLION)
TABLE 39. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ASSEMBLIES, BY REGION, 2018-2024 (USD MILLION)
TABLE 40. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ASSEMBLIES, BY REGION, 2025-2030 (USD MILLION)
TABLE 41. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY BAGS, BY REGION, 2018-2024 (USD MILLION)
TABLE 42. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY BAGS, BY REGION, 2025-2030 (USD MILLION)
TABLE 43. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 44. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 45. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 46. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 47. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY HIGH HARDNESS, BY REGION, 2018-2024 (USD MILLION)
TABLE 48. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY HIGH HARDNESS, BY REGION, 2025-2030 (USD MILLION)
TABLE 49. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MEDIUM HARDNESS, BY REGION, 2018-2024 (USD MILLION)
TABLE 50. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MEDIUM HARDNESS, BY REGION, 2025-2030 (USD MILLION)
TABLE 51. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SOFT HARDNESS, BY REGION, 2018-2024 (USD MILLION)
TABLE 52. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SOFT HARDNESS, BY REGION, 2025-2030 (USD MILLION)
TABLE 53. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 54. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 55. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, BY REGION, 2018-2024 (USD MILLION)
TABLE 56. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, BY REGION, 2025-2030 (USD MILLION)
TABLE 57. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY THREE LAYER, BY REGION, 2018-2024 (USD MILLION)
TABLE 58. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY THREE LAYER, BY REGION, 2025-2030 (USD MILLION)
TABLE 59. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY TWO LAYER, BY REGION, 2018-2024 (USD MILLION)
TABLE 60. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY TWO LAYER, BY REGION, 2025-2030 (USD MILLION)
TABLE 61. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 62. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 63. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY EXTRUSION, BY REGION, 2018-2024 (USD MILLION)
TABLE 64. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY EXTRUSION, BY REGION, 2025-2030 (USD MILLION)
TABLE 65. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 66. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 67. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY AUTOCLAVE, BY REGION, 2018-2024 (USD MILLION)
TABLE 68. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY AUTOCLAVE, BY REGION, 2025-2030 (USD MILLION)
TABLE 69. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY E BEAM, BY REGION, 2018-2024 (USD MILLION)
TABLE 70. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY E BEAM, BY REGION, 2025-2030 (USD MILLION)
TABLE 71. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ETHYLENE OXIDE, BY REGION, 2018-2024 (USD MILLION)
TABLE 72. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ETHYLENE OXIDE, BY REGION, 2025-2030 (USD MILLION)
TABLE 73. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY GAMMA, BY REGION, 2018-2024 (USD MILLION)
TABLE 74. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY GAMMA, BY REGION, 2025-2030 (USD MILLION)
TABLE 75. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 76. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 77. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY AFTERMARKET DISTRIBUTION, BY REGION, 2018-2024 (USD MILLION)
TABLE 78. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY AFTERMARKET DISTRIBUTION, BY REGION, 2025-2030 (USD MILLION)
TABLE 79. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DIRECT SALES, BY REGION, 2018-2024 (USD MILLION)
TABLE 80. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DIRECT SALES, BY REGION, 2025-2030 (USD MILLION)
TABLE 81. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY OEM, BY REGION, 2018-2024 (USD MILLION)
TABLE 82. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY OEM, BY REGION, 2025-2030 (USD MILLION)
TABLE 83. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ONLINE DISTRIBUTION, BY REGION, 2018-2024 (USD MILLION)
TABLE 84. GLOBAL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY ONLINE DISTRIBUTION, BY REGION, 2025-2030 (USD MILLION)
TABLE 85. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 86. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 87. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 88. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 89. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 90. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 91. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 92. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 93. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 94. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 95. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 96. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 97. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 98. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 99. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 100. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 101. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 102. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 103. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 104. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 105. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2018-2024 (USD MILLION)
TABLE 106. AMERICAS BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2025-2030 (USD MILLION)
TABLE 107. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 108. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 109. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 110. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 111. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 112. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 113. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 114. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 115. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 116. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 117. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 118. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 119. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 120. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 121. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 122. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 123. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 124. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 125. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 126. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 127. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STATE, 2018-2024 (USD MILLION)
TABLE 128. UNITED STATES BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STATE, 2025-2030 (USD MILLION)
TABLE 129. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 130. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 131. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 132. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 133. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 134. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 135. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 136. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 137. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 138. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 139. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 140. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 141. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 142. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 143. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 144. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 145. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 146. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 147. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 148. CANADA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 149. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 150. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 151. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 152. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 153. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 154. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 155. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 156. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 157. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 158. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 159. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 160. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 161. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 162. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 163. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 164. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 165. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 166. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 167. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 168. MEXICO BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 169. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 170. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 171. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 172. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 173. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 174. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 175. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 176. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 177. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 178. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 179. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 180. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 181. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 182. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 183. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 184. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 185. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 186. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 187. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 188. BRAZIL BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 189. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 190. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 191. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 192. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 193. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 194. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 195. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 196. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 197. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 198. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 199. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 200. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 201. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 202. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 203. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 204. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 205. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 206. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 207. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 208. ARGENTINA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 209. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 210. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 211. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 212. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 213. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 214. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 215. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 216. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 217. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 218. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 219. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 220. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 221. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 222. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 223. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 224. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 225. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 226. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 227. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 228. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 229. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2018-2024 (USD MILLION)
TABLE 230. EUROPE, MIDDLE EAST & AFRICA BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY COUNTRY, 2025-2030 (USD MILLION)
TABLE 231. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 232. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 233. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 234. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 235. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 236. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 237. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 238. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 239. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 240. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 241. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 242. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 243. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 244. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 245. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 246. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 247. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 248. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 249. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 250. UNITED KINGDOM BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 251. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 252. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 253. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 254. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 255. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 256. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 257. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 258. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 259. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 260. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 261. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 262. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 263. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 264. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 265. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 266. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 267. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 268. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2025-2030 (USD MILLION)
TABLE 269. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2024 (USD MILLION)
TABLE 270. GERMANY BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY DISTRIBUTION CHANNEL, 2025-2030 (USD MILLION)
TABLE 271. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2018-2024 (USD MILLION)
TABLE 272. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY APPLICATION, 2025-2030 (USD MILLION)
TABLE 273. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2018-2024 (USD MILLION)
TABLE 274. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CHROMATOGRAPHY, 2025-2030 (USD MILLION)
TABLE 275. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2018-2024 (USD MILLION)
TABLE 276. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FILTRATION, 2025-2030 (USD MILLION)
TABLE 277. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2018-2024 (USD MILLION)
TABLE 278. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY FLUID TRANSFER, 2025-2030 (USD MILLION)
TABLE 279. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2018-2024 (USD MILLION)
TABLE 280. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY SINGLE USE SYSTEM, 2025-2030 (USD MILLION)
TABLE 281. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2018-2024 (USD MILLION)
TABLE 282. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MATERIAL GRADE, 2025-2030 (USD MILLION)
TABLE 283. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2018-2024 (USD MILLION)
TABLE 284. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY MANUFACTURING PROCESS, 2025-2030 (USD MILLION)
TABLE 285. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2018-2024 (USD MILLION)
TABLE 286. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY CO-EXTRUSION, 2025-2030 (USD MILLION)
TABLE 287. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STERILIZATION METHOD, 2018-2024 (USD MILLION)
TABLE 288. FRANCE BIOPHARMACEUTICAL GRADE TPE TUBING MARKET SIZE, BY STE
Companies Mentioned
- Avantor, Inc.
- Avient Corporation
- Compagnie de Saint-Gobain SA
- DuPont de Nemours, Inc.
- Eldon James Corporation
- Freudenberg SE
- IDEX Health & Science LLC
- Kent Elastomer Products, Inc.
- Meissner Filtration Products, Inc.
- Nordson Corporation
- Parker-Hannifin Corporation
- Qosina Corp.
- Raumedic AG
- Saint-Gobain Life Sciences
- SaniSure, Inc.
- Sartorius AG
- Spirax-Sarco Engineering plc
- Tekni-Plex, Inc.
- Teknor Apex Company
- Trelleborg AB
- Watson-Marlow Fluid Technology Group
- Zeus Industrial Products, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 723.59 Million |
| Forecasted Market Value ( USD | $ 1280 Million |
| Compound Annual Growth Rate | 10.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |