Speak directly to the analyst to clarify any post sales queries you may have.
Botulinum toxin in medical cosmetology is entering a precision-driven era where patient expectations, safety scrutiny, and operational rigor define success
Botulinum toxin has moved from a niche aesthetic intervention to a foundational modality in medical cosmetology, valued for its predictable onset, procedural efficiency, and broad applicability across age groups and indications. While the category is still popularly associated with wrinkle reduction, clinical practice has expanded into facial balancing, preventive treatment among younger cohorts, and combination protocols that pair neuromodulators with energy-based devices and dermal fillers. This evolution reflects a larger recalibration in aesthetic medicine: outcomes are increasingly judged on natural movement, individualized dosing, and patient experience rather than on maximal line erasure.At the same time, the industry is operating under a more complex set of constraints. Provider capacity, injector competency, pharmacovigilance, product authenticity, and cold-chain integrity now materially influence both results and reputational risk. As clinics compete on service quality and safety, manufacturers and distributors are pressured to deliver consistency-across lots, geographies, and logistics conditions-while supporting education and evidence generation. In parallel, patients are behaving more like informed consumers, arriving with reference images, brand preferences, and expectations for transparent aftercare.
Against this backdrop, decision-makers across manufacturers, specialty distributors, clinic networks, and training organizations are prioritizing resilience and differentiation. The executive perspective for botulinum toxin in medical cosmetology, therefore, must reconcile clinical nuance with operational realities, including evolving regulations, cross-border trade pressures, and shifting competitive tactics. The sections that follow synthesize the most consequential changes shaping performance and risk in 2025 and beyond, helping stakeholders align strategy with the new rules of aesthetic practice.
Clinical protocols, authenticity safeguards, and stricter governance are redefining competitive advantage in the evolving botulinum toxin ecosystem
The landscape for botulinum toxin in medical cosmetology is being reshaped by a decisive shift from “procedure-first” thinking to “protocol-first” care. Clinics are standardizing assessment frameworks that account for facial dynamics, muscle strength, and patient lifestyle factors, which in turn raises the value of training, documentation, and outcome tracking. As a result, product choice is increasingly influenced not only by brand familiarity but also by how reliably a toxin integrates into reproducible protocols, including reconstitution preferences, dosing philosophies, and follow-up cadence.Another transformative shift is the growing importance of authenticity and supply-chain transparency. Counterfeit and diverted products remain a persistent risk in global aesthetics, and heightened awareness has pushed reputable providers toward authorized channels and verification practices. This has elevated the strategic role of specialty distributors and clinic procurement teams, who now evaluate vendors on more than price-seeking validated chain-of-custody, temperature control, and responsive recall procedures. In parallel, manufacturers are investing in packaging security features and education that helps clinics recognize red flags before patient safety is compromised.
Consumer behavior is also changing the competitive equation. Patients increasingly expect “soft-touch” results with minimal downtime, and they compare outcomes across social platforms and peer networks. This social visibility rewards providers who excel at natural aesthetics, but it also penalizes inconsistent technique. Consequently, demand is accelerating for structured injector education, mentorship models, and brand-supported training ecosystems that can scale best practices across multi-site clinic groups.
Finally, regulatory and ethical expectations are tightening. Jurisdictions are revisiting who can inject, what supervision is required, and how adverse events must be documented and reported. Even where formal rules are stable, malpractice insurers and professional boards are exerting de facto pressure toward higher standards. Taken together, these shifts are transforming botulinum toxin from a high-volume cosmetic service into a clinically governed, brand-sensitive, and compliance-intensive offering where operational discipline becomes a competitive moat.
Potential 2025 U.S. tariff pressure could reshape procurement, logistics, and clinic economics, making resilience planning a strategic necessity
United States tariff actions expected to take effect or expand in 2025 introduce a meaningful layer of cost and planning complexity for the botulinum toxin value chain, even when the active product itself is not the direct tariff target. Medical cosmetology depends on a wide set of imported inputs-specialty syringes, needles, cold-chain packaging materials, refrigerated logistics equipment, and certain device-adjacent consumables used in combined aesthetic protocols. When tariffs raise acquisition costs for these supporting components, clinics and distributors may face higher per-procedure overhead, creating pressure to adjust pricing, reduce waste, or renegotiate supplier terms.In response, procurement strategies are likely to become more conservative and more diversified. Distributors may rebalance inventory toward suppliers with domestic warehousing and more predictable lead times, while larger clinic groups may centralize purchasing to secure consistent pricing and reduce exposure to spot-market volatility. At the manufacturer level, tariff-linked uncertainty can encourage localized packaging, secondary assembly, or alternative sourcing of non-active components to protect margins and preserve continuity. However, qualification and validation requirements in healthcare logistics mean that switching suppliers is rarely immediate; therefore, transition plans must account for regulatory documentation, stability considerations, and quality audits.
The cumulative impact also extends to access and equity. If operating costs rise, smaller practices may be less able to absorb margin compression, potentially widening the gap between premium clinic networks and independent providers. Meanwhile, patients could become more selective in treatment cadence or shift toward fewer, higher-trust providers, reinforcing the importance of retention and membership-style programs that smooth demand. Importantly, tariff-related cost pressure may also accelerate interest in operational efficiencies such as optimized appointment scheduling, standardized dosing to minimize reconstitution waste, and tighter inventory controls.
Ultimately, the most resilient organizations will treat tariffs not as a one-time pricing event but as a catalyst to modernize sourcing governance and scenario planning. Those that proactively map bill-of-materials exposure, qualify backup suppliers, and strengthen distributor collaboration will be better positioned to maintain service consistency while competitors react defensively.
Segmentation patterns show repeatable outcomes, scalable clinic models, and channel trust becoming the real drivers of differentiation and retention
Segmentation reveals that the market’s strategic center of gravity is shifting toward use cases and delivery models that prioritize repeatability, patient trust, and provider control. Product type differentiation increasingly matters in day-to-day clinic operations because formulation characteristics influence handling, storage routines, and how injectors calibrate outcomes over time. In practice, clinics often align specific products to signature looks and standardized protocols, which makes consistency across visits a central retention lever. This dynamic is particularly influential as multi-site clinic groups expand and seek to replicate results across locations without diluting brand identity.From an application perspective, the strongest strategic pull comes from indications where outcomes are both visible and reliably repeatable, supporting predictable revisit cycles and high patient satisfaction. However, the category is also benefiting from adjacent medical-cosmetic overlaps, where patients perceive both aesthetic and wellness value. As providers incorporate botulinum toxin into broader facial harmonization plans, complementary treatments influence when and how toxin is used, shifting demand toward integrated care pathways rather than isolated injections.
End-user segmentation underscores widening separation between high-throughput medspas, physician-led dermatology practices, and plastic surgery settings, each with distinct economics and risk tolerance. Medspas compete on experience, convenience, and service bundles, which makes training systems and standardized workflows decisive. Dermatology and plastic surgery clinics often emphasize medical credibility, complex case handling, and combination procedures, shaping preferences for products, dosing philosophies, and patient education depth. Meanwhile, the rise of organized clinic networks and corporate-backed platforms is professionalizing procurement and compliance, often favoring vendors that can support multi-location governance, credential tracking, and consistent supply.
Distribution channel segmentation further highlights the premium placed on authenticity and continuity. Authorized distribution is becoming a strategic differentiator as clinics seek to reduce counterfeit exposure and streamline cold-chain assurance. At the same time, evolving buying behavior is pushing some organizations toward consolidated purchasing and longer-term agreements to stabilize costs and reduce operational noise. In combination, these segmentation dynamics indicate that growth and defensibility are increasingly linked to how well a stakeholder supports repeatable clinical outcomes, scalable training, and low-friction procurement rather than to brand awareness alone.
Regional dynamics reveal how regulation, cultural aesthetics, and clinic infrastructure shape adoption differently across major geographies worldwide
Regional dynamics in botulinum toxin medical cosmetology are diverging based on regulatory posture, consumer aesthetics preferences, and healthcare delivery structure. In the Americas, demand is strongly influenced by professionalization of injectables within clinic networks and by consumer emphasis on subtle, natural-looking results. The region’s competitive intensity rewards providers who pair strong consultation practices with consistent outcomes, and it places added focus on authenticity and standardized training as multi-site models expand.In Europe, Middle East & Africa, country-level regulatory variation remains a defining feature, affecting who can inject, how products are procured, and how advertising is constrained. This creates a landscape where compliance maturity and clinical governance can be a stronger differentiator than promotional reach. At the same time, established aesthetic hubs tend to adopt advanced combination protocols more quickly, raising the importance of cross-disciplinary expertise and careful patient selection.
Across Asia-Pacific, a blend of rapidly evolving consumer demand and diverse regulatory environments shapes adoption. In several markets, beauty standards and cultural preferences influence injection patterns, driving tailored approaches to dosing and facial balancing. The region also exhibits strong momentum in aesthetic services infrastructure, with clinic expansion and technology adoption supporting broader access. However, the same growth can heighten the need for robust training ecosystems and supply-chain controls to protect patient safety and provider reputation.
Taken together, regional insights point to a common lesson: strategy must be localized without fragmenting operational standards. Organizations that can adapt to local compliance, cultural aesthetics, and channel realities-while maintaining consistent product integrity, training quality, and documentation-are more likely to build durable presence across regions.
Company differentiation now depends on education ecosystems, authenticity assurance, and clinic-operational support that protects outcomes and reputation
Competition among leading companies in botulinum toxin medical cosmetology is increasingly centered on trust, education, and operational support rather than on brand recognition alone. Established players leverage long-standing clinician relationships, published evidence, and broad field education to reinforce confidence in predictable outcomes. Their strategies commonly emphasize injector training, complication management guidance, and practice development tools that help clinics improve consultation quality, retention, and patient communication.At the same time, differentiation is intensifying through service models that surround the vial. Companies are investing in programs that support clinic workflow standardization, digital patient engagement, and loyalty structures that encourage repeat visits. In markets where price sensitivity is rising, manufacturers and distributors are also refining contracting approaches, including volume-based agreements and structured purchasing programs that help multi-site providers stabilize supply while maintaining compliance.
Innovation remains important, but it is increasingly evaluated through the lens of real-world practicality. Providers value stability in handling, clarity of labeling, and support resources that reduce chair-time variability. As combination treatments become more common, companies that can position their toxin within an integrated aesthetic plan-through education on facial assessment, sequencing with fillers or devices, and patient counseling-tend to strengthen clinical mindshare.
Finally, corporate behavior regarding safety and authenticity is now a competitive signal. Organizations that actively communicate anti-counterfeit measures, maintain responsive medical affairs engagement, and provide transparent guidance on adverse event reporting are better positioned to earn long-term clinic loyalty. In an environment where reputational risk can spread quickly, the companies perceived as the safest partners often gain an advantage that extends beyond product performance alone.
Leaders who standardize care, secure supply chains, and institutionalize injector education will outperform amid volatility and rising patient scrutiny
Industry leaders can take immediate steps to strengthen competitiveness by treating clinical consistency as an enterprise capability rather than an individual injector skill. Standardizing assessment, dosing documentation, and follow-up routines across locations reduces outcome variability and makes training more scalable. This approach also supports better patient communication, since expectations are set using repeatable language and measurable goals rather than subjective promises.Next, organizations should harden supply-chain governance with explicit authenticity and cold-chain controls. That means tightening vendor qualification, insisting on documented chain-of-custody, and implementing intake checks that verify packaging integrity and lot traceability. In parallel, strengthening inventory discipline-such as aligning order quantities with appointment scheduling and minimizing reconstitution waste-can protect margins when input costs fluctuate.
Leaders should also build a structured education flywheel that combines onboarding, supervised practice, and continuing education tied to complication readiness. Training that addresses facial dynamics, patient selection, and aftercare reduces adverse events and improves satisfaction, which directly supports retention. Additionally, investing in patient experience design-consultation flow, informed consent clarity, and post-treatment touchpoints-can differentiate clinics in a crowded field where consumers increasingly reward professionalism and transparency.
Finally, strategic planning should incorporate tariff and trade uncertainty as an ongoing scenario rather than a temporary disruption. Mapping exposure across consumables and logistics, pre-qualifying alternative suppliers, and aligning with distribution partners on buffer stock policies can reduce service interruptions. Organizations that couple these operational moves with disciplined brand positioning-emphasizing natural results and safety-will be better equipped to sustain performance under shifting economic and regulatory conditions.
A structured methodology blending expert interviews, regulatory review, and triangulated validation delivers clinically grounded, decision-ready insights
This research methodology is designed to produce decision-grade insight into botulinum toxin use in medical cosmetology by combining structured primary inputs with rigorous secondary validation. The process begins with a detailed mapping of the value chain, including manufacturers, authorized distributors, clinic networks, and training organizations, to clarify how product moves through regulated channels and how service delivery influences demand patterns.Primary research emphasizes expert interviews with stakeholders such as practicing injectors, clinic managers, procurement leads, and industry advisors. These conversations focus on treatment protocols, product handling preferences, patient decision drivers, adverse event management practices, and the operational impact of evolving regulation. Insights are synthesized to identify common patterns as well as points of divergence by practice type and geography, ensuring conclusions reflect real-world clinical workflows.
Secondary research consolidates information from regulatory publications, professional association materials, scientific and clinical literature, corporate disclosures, and trade documentation relevant to medical aesthetics logistics. This evidence is used to triangulate themes identified in interviews, confirm terminology and compliance requirements, and track industry shifts such as anti-counterfeit measures, training standards, and changes in distribution practices.
Analytical steps include segmentation-based synthesis, cross-regional comparison, and qualitative competitive assessment focused on capabilities and positioning. Throughout, quality control procedures are applied to reduce bias, reconcile conflicting inputs, and ensure that findings are internally consistent, clinically plausible, and operationally actionable for decision-makers.
The market’s next phase will reward organizations that pair clinical excellence with resilient operations, compliance maturity, and patient trust
Botulinum toxin in medical cosmetology is advancing into a phase where execution quality matters as much as product choice. Providers are expected to deliver natural outcomes with high consistency, supported by transparent consent, strong aftercare, and well-governed clinical protocols. Meanwhile, manufacturers and distributors are being evaluated on authenticity assurance, educational support, and supply continuity as much as on brand reputation.As competitive intensity rises, the market is also becoming more sensitive to external pressures such as tariffs, logistics costs, and regulatory scrutiny over injector qualifications. These forces reward organizations that invest in operational discipline-standardized workflows, training systems, and resilient procurement-because such capabilities protect both patient outcomes and business stability.
Across regions, localized adaptation is essential, but it must be balanced with consistent standards for safety, documentation, and product integrity. Stakeholders that can align clinical excellence with supply-chain rigor and patient-centric experience design will be best positioned to build trust, sustain retention, and navigate uncertainty without compromising care quality.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Botulinum Toxin in Medical Cosmetology Market
Companies Mentioned
The key companies profiled in this Botulinum Toxin in Medical Cosmetology market report include:- AbbVie Inc.
- Croma-Pharma GmbH
- Daewoong Pharmaceutical Co., Ltd.
- Eisai Co., Ltd.
- Evolus, Inc.
- Galderma S.A.
- Gufic BioSciences Limited
- Hugel, Inc.
- Huons Global Co., Ltd.
- Ipsen Pharma S.A.S.
- Lanzhou Institute of Biological Products Co., Ltd.
- Medytox, Inc.
- Merz Pharma GmbH & Co. KGaA
- Revance Therapeutics, Inc.
- Shanghai Fosun Pharmaceutical (Group) Co., Ltd.
- Supernus Pharmaceuticals, Inc.
- US WorldMeds, LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
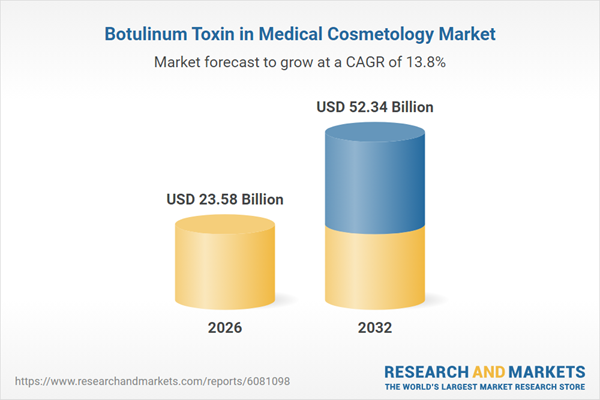
| Estimated Market Value ( USD | $ 23.58 Billion |
| Forecasted Market Value ( USD | $ 52.34 Billion |
| Compound Annual Growth Rate | 13.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |