Speak directly to the analyst to clarify any post sales queries you may have.
Aspiration-driven thrombectomy is redefining procedural expectations, making negative pressure pumps a focal point for outcomes, workflow, and value
Thrombectomy has become a cornerstone intervention across multiple acute vascular emergencies, and aspiration-assisted techniques have gained meaningful traction as clinicians seek faster reperfusion, reduced clot burden, and streamlined procedural steps. Within this environment, the thrombectomy aspiration negative pressure pump has moved from being a peripheral accessory to a highly scrutinized enabling technology, because it directly influences suction stability, catheter compatibility, operator ergonomics, and the consistency of aspiration performance during high-stakes cases.At the same time, decision-makers are treating aspiration pumps as part of a broader procedural ecosystem that includes aspiration catheters, tubing sets, canisters, hemostasis valves, and imaging guidance. As a result, purchasing teams and clinicians increasingly evaluate pumps not only on raw vacuum performance, but also on controllability, reliability under continuous use, ease of setup, sterility pathways, noise and footprint constraints in crowded suites, and how seamlessly the pump integrates with existing thrombectomy workflows.
Against this backdrop, manufacturers face a dual imperative. They must deliver clinically meaningful performance improvements-such as responsive vacuum modulation and minimized flow disruptions-while also meeting tightening expectations around quality systems, post-market surveillance, and total cost of ownership. This executive summary frames the most important forces shaping adoption and competition, highlighting how technology evolution, policy dynamics, segmentation behavior, and regional operating realities are redefining what “best-in-class” looks like for negative pressure aspiration in thrombectomy.
From simple vacuum generation to controlled, system-integrated performance, aspiration pump innovation is reshaping clinical workflows and competition
Technology development in aspiration pumping is shifting from basic vacuum generation toward precise control and workflow intelligence. Clinicians increasingly expect stable suction with the option to modulate vacuum rapidly, minimizing sudden pressure drops that can reduce aspiration efficiency or increase the risk of catheter clogging. Consequently, product differentiation is moving into domains such as responsive pressure control, user interfaces that support intuitive adjustments mid-procedure, and alarm systems that improve situational awareness without adding cognitive burden.In parallel, the market is being reshaped by a broader shift toward system-level solutions. Instead of evaluating pumps as standalone devices, providers are assessing complete thrombectomy solutions that include aspiration catheters, stent retrievers, access systems, and compatible disposables. This bundling dynamic is accelerating as hospitals seek procurement simplification and standardized clinical pathways. For pump suppliers, it raises the bar on interoperability and drives partnerships or portfolio expansion to ensure that the pump is not excluded from comprehensive contracts.
Another transformative shift is the increasing attention to evidence generation and real-world performance consistency. Health systems and clinicians are looking beyond short-term technical claims, focusing on repeatability across varied anatomies and clot types, and across different operator skill levels. This strengthens the value of robust usability engineering, training support, and post-market feedback loops that can inform iterative improvements.
Finally, supply chain resilience has emerged as a strategic differentiator rather than a back-office concern. Procurement teams want assurance around component availability, serviceability, and the continuity of consumables. Manufacturers that can demonstrate multi-sourcing strategies, strong quality oversight for contract manufacturing, and responsive field service are better positioned to win long-term placements, particularly in environments where procedural volume is unpredictable and downtime is unacceptable.
United States tariff dynamics in 2025 are pushing aspiration pump makers toward redesign, sourcing resilience, and clearer procurement value narratives
The 2025 tariff environment in the United States is expected to influence thrombectomy aspiration negative pressure pump strategies in ways that go beyond headline import costs. Because pumps often rely on globally sourced components-such as motors, sensors, control boards, plastics, and sterile pathway interfaces-tariff exposure can appear in both finished devices and subassemblies. This creates a cascading effect in bill-of-materials planning, qualification timelines, and service-part inventories.In response, many suppliers are likely to intensify tariff engineering: redesigning assemblies to reduce exposure, adjusting country-of-origin configurations, and reassessing contract manufacturing footprints. However, these actions can introduce validation and documentation burdens, especially in regulated environments where even minor component changes may require verification, risk reassessment, and sometimes regulatory notifications. Therefore, tariff-driven redesign can become a product development constraint, potentially diverting engineering resources from performance innovation unless carefully managed.
On the demand side, tariffs can alter procurement behavior by encouraging longer contract durations, earlier purchasing cycles, or preference for suppliers with domestic assembly and stronger predictability in lead times. Hospitals and group purchasing stakeholders may also seek clearer breakdowns of disposable costs tied to the pump ecosystem, because tubing and canisters can be more sensitive to frequent procurement cycles than capital equipment.
Over time, the cumulative impact is likely to favor companies that treat trade policy volatility as a recurring operating condition. Those with dual-region manufacturing options, disciplined supplier qualification, and transparent pricing communication are better able to maintain trust with providers. Meanwhile, companies that rely heavily on single-region sourcing may face margin pressure or be forced into abrupt price adjustments, which can weaken competitive positioning during contract renewals.
Segmentation reveals that pump selection hinges on product design, pressure controllability, application urgency, end-user workflow, and channel support depth
Segmentation behavior in thrombectomy aspiration negative pressure pumps is increasingly defined by how clinical settings translate procedural risk into purchasing requirements. When viewed through the lens of product type, decision criteria diverge between compact solutions favored for rapid setup and space-limited suites and more feature-rich platforms prioritized where complex cases and higher volumes justify enhanced control capabilities. This distinction becomes more pronounced as hospitals seek standardization; a single platform may not optimally serve every care setting without configuration flexibility.Differences also sharpen across pressure range expectations. Some users prioritize high and sustained negative pressure for dense thrombus management, while others emphasize smooth vacuum stability and the ability to titrate suction to reduce unwanted vessel wall interactions. As aspiration techniques evolve, the value of precision and repeatability is rising, and buyers increasingly ask whether performance remains stable across prolonged aspiration and partial occlusion scenarios.
The application lens reveals that clinical workflows and urgency profiles shape feature preferences. In time-critical environments, setup speed, intuitive controls, and dependable alarms can matter as much as peak suction. In settings where aspiration is used alongside other mechanical strategies, interoperability and predictable catheter behavior become the focus, because operators may switch techniques quickly depending on clot response.
Procurement and utilization patterns further diverge by end user context. High-volume centers tend to emphasize durability, service response, and training support, while smaller facilities may prioritize ease of use, minimal maintenance demands, and predictable consumable availability. The ability to integrate with existing room layouts and infection control protocols can be decisive, particularly when staff rotate frequently.
Finally, distribution channel realities shape adoption speed and lifecycle support. Direct sales models can accelerate clinical education and protocol adoption, whereas distributor-led models may expand geographic reach but require strong partner enablement to ensure consistent installation, maintenance, and troubleshooting. Across segments, the strongest offerings are those that align performance claims with the practical constraints of staffing, sterility pathways, and total workflow time.
Regional adoption varies with care infrastructure and procurement models, requiring tailored evidence, service, and channel strategies across global markets
Regional dynamics are shaped by differences in stroke systems of care, vascular intervention infrastructure, reimbursement complexity, and procurement governance. In Americas, demand is strongly influenced by comprehensive stroke center networks, competitive contracting, and heightened scrutiny of total procedure economics. Providers often expect strong clinical support, rapid service turnaround, and clear integration with established thrombectomy toolkits, which raises the premium on training programs and predictable consumable logistics.Across Europe, Middle East & Africa, adoption patterns reflect the diversity of health system structures and tendering approaches. In many European markets, formal procurement processes and emphasis on documented performance and safety can elongate adoption cycles, while also rewarding suppliers that can provide robust technical files, standardized training, and long-term service commitments. Within parts of the Middle East, investment in advanced intervention capability can accelerate technology uptake, although supplier qualification and service coverage remain critical. In several African settings, access is often constrained by infrastructure variability and budget limits, shifting emphasis toward reliability, simplified maintenance, and the availability of compatible disposables.
In Asia-Pacific, growth in neurointerventional capacity and expanding specialist training are key demand catalysts, but the region remains heterogeneous. Advanced markets may prioritize sophisticated control features and workflow optimization, while rapidly developing markets can place greater weight on cost discipline, distributor capability, and scalability of training. Across the region, local regulatory pathways and domestic manufacturing initiatives can influence supplier strategy, encouraging partnerships, localization, and tailored after-sales models.
Taken together, regional insights underscore that a single global playbook is rarely sufficient. Suppliers that adapt evidence messaging, service design, and channel execution to the operational realities of each region are better positioned to build durable placements and expand procedural adoption responsibly.
Competitive advantage increasingly comes from combining suction performance with consumables execution, interoperability, service quality, and regulatory readiness
Competitive positioning in thrombectomy aspiration negative pressure pumps increasingly depends on how well companies combine device engineering with ecosystem execution. Leading players distinguish themselves by delivering stable suction performance while reducing setup friction, supporting compatibility across commonly used aspiration catheters, and reinforcing trust through dependable service and training. As clinicians place greater emphasis on repeatability, manufacturers that can demonstrate consistent pump behavior under real procedural conditions gain an advantage.Another differentiator lies in how companies manage consumables and lifecycle support. Even when the pump is a capital asset, day-to-day satisfaction often hinges on the steady availability of tubing sets, canisters, connectors, and sterile accessories. Companies that align their consumables strategy with hospital inventory practices-and that provide clear guidance on replacement intervals, cleaning protocols where applicable, and troubleshooting-tend to achieve stronger retention.
Strategically, partnerships and portfolio breadth are shaping procurement outcomes. Suppliers with adjacent thrombectomy devices or strong alliances can present a more complete procedural offering, which resonates with health systems looking to simplify vendor management and standardize training. At the same time, focused specialists can compete effectively by excelling in usability, service responsiveness, and delivering a strong clinical education model that helps clinicians realize the pump’s benefits quickly.
Finally, quality systems maturity and regulatory readiness are becoming more visible to buyers. Hospitals and purchasing organizations increasingly seek assurance that manufacturers can manage component changes, field corrections, and post-market monitoring with minimal disruption. Companies that proactively communicate quality practices and provide transparent documentation are better positioned to sustain long-term contracts in an environment where risk management is tightly linked to patient safety.
Leaders can win through controllable performance, consumables excellence, resilient sourcing, and training-driven value narratives aligned to real workflows
Industry leaders can strengthen their position by prioritizing controllability and usability as core product themes rather than secondary refinements. Investing in responsive vacuum modulation, clear interfaces, and procedure-friendly alarm logic can reduce operator variability and support consistent performance across diverse case types. In parallel, aligning the pump’s physical design with real suite constraints-noise, footprint, and cable management-can meaningfully improve day-to-day adoption.Next, companies should treat consumables strategy as a primary growth lever. Ensuring reliable availability of tubing and collection components, simplifying ordering, and designing packaging that supports rapid room turnover can increase customer satisfaction as much as device features. Where possible, standardizing connectors and validating compatibility with widely used aspiration catheters can reduce friction for hospitals that operate mixed-vendor environments.
Given the tariff and supply volatility backdrop, leaders should also harden sourcing and manufacturing resilience. Dual sourcing for high-risk components, disciplined change control, and pre-validated alternates can reduce disruption when trade conditions shift. Additionally, transparent communication with procurement teams about lead times, service coverage, and pricing logic can preserve trust during contract negotiations.
Finally, commercial execution should emphasize evidence and training that map directly to clinical workflows. Structured onboarding, simulation-based support, and clear protocols for managing clogging or pressure instability help clinicians realize value quickly. By linking product claims to measurable workflow improvements-such as reduced setup time or fewer interruptions-companies can strengthen their value narrative without relying on broad generalizations.
A triangulated methodology combining stakeholder interviews and validated documentation builds a dependable view of technology, procurement, and clinical needs
The research methodology for this analysis integrates structured primary engagement with rigorous secondary review to capture both clinical realities and commercial decision drivers. Primary inputs typically include interviews with stakeholders such as interventional clinicians, cath lab and neurointerventional staff, biomedical engineering teams, procurement leaders, and distribution partners. These conversations are used to validate how aspiration pumps are evaluated in practice, what features matter during procedures, and which service and consumables factors most influence renewal decisions.Secondary research consolidates publicly available and authoritative materials such as regulatory databases and filings, manufacturer product documentation, quality and safety communications where accessible, clinical guideline updates, peer-reviewed literature on thrombectomy techniques, and publicly disclosed company information related to operations and partnerships. This helps ensure that the analysis reflects current device requirements and technology directions while remaining grounded in verifiable information.
Triangulation is applied throughout to reconcile differences between stakeholder perspectives and documented evidence. Findings are stress-tested by comparing reported purchasing behavior with observed procurement mechanisms, and by checking technical claims against device documentation and regulatory expectations. Finally, the market framework is organized around segmentation and regional lenses to ensure that insights remain actionable for product, commercial, and sourcing teams.
This approach is designed to surface not only what is changing, but why it is changing, and what that implies for strategy-helping readers connect clinical needs, policy realities, and operational constraints into coherent decisions.
Aspiration pump success now depends on controllable performance, dependable lifecycle support, and resilient operations aligned with evolving thrombectomy practice
Thrombectomy aspiration negative pressure pumps are gaining strategic importance as aspiration-assisted techniques mature and providers demand more predictable, controllable performance. What was once judged primarily by vacuum strength is now evaluated through a wider lens that includes usability, interoperability, consumables reliability, and the ability to support consistent outcomes across varied clinical scenarios.As the competitive landscape evolves, system-level selling, evidence expectations, and after-sales execution increasingly determine which pumps become standardized within health systems. Meanwhile, trade and supply uncertainties add pressure to build resilient manufacturing and sourcing strategies without compromising regulatory compliance or product quality.
The most successful participants will be those that align engineering priorities with real procedural workflows, reinforce value through training and service, and tailor regional go-to-market approaches to procurement structures and care infrastructure. In doing so, they can convert technical capability into durable adoption and long-term customer trust.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Thrombectomy Aspiration Negative Pressure Pump Market
Companies Mentioned
The key companies profiled in this Thrombectomy Aspiration Negative Pressure Pump market report include:- Abbott Laboratories
- Acandis GmbH & Co. KG
- AngioDynamics, Inc.
- Becton, Dickinson and Company
- Boston Scientific Corporation
- Cook Medical LLC
- Imperative Care, Inc.
- Inari Medical, Inc.
- Medtronic plc
- Merit Medical Systems, Inc.
- NeuroVasc Technologies, Inc.
- Nico Corporation
- Penumbra, Inc.
- Route 92 Medical, Inc.
- Shandong Weigao Group Medical Polymer Co., Ltd.
- Stryker Corporation
- Teleflex Incorporated
- Terumo Corporation
- Vesalio, LLC
- Wallaby Medical, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 191 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
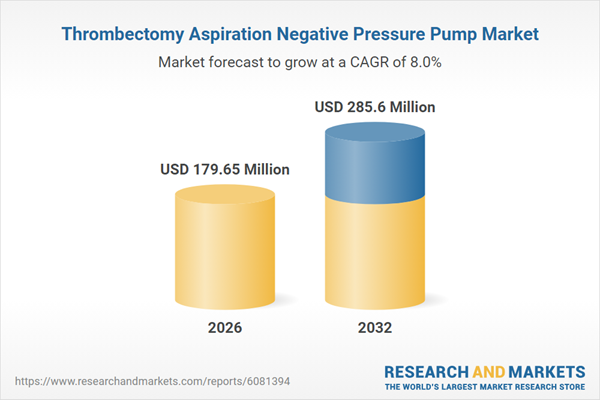
| Estimated Market Value ( USD | $ 179.65 Million |
| Forecasted Market Value ( USD | $ 285.6 Million |
| Compound Annual Growth Rate | 8.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |