Speak directly to the analyst to clarify any post sales queries you may have.
The Biostorage Market is undergoing significant change as the need for secure and compliant sample storage intensifies across research, clinical, and industrial sectors. Senior executives must address rising operational and regulatory demands by adopting agile strategies and prioritizing innovative storage solutions that support both growth and risk management.
Market Snapshot: Biostorage Market Growth and Opportunity
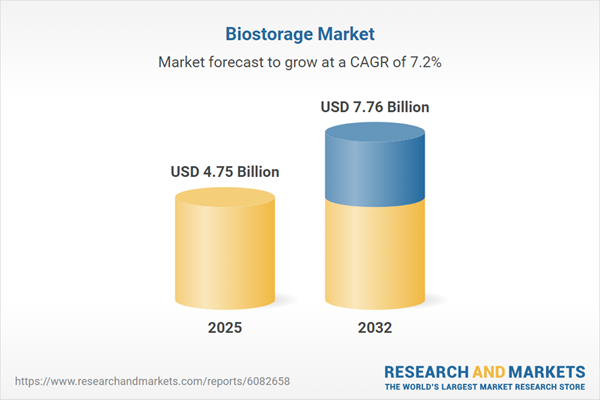
The biostorage market is demonstrating robust expansion, with market value projected to rise from USD 4.44 billion in 2024 to USD 4.75 billion in 2025, and a forecast compound annual growth rate of 7.21%. By 2032, the market is anticipated to reach USD 7.76 billion. Growth is propelled by the surging demand for reliable, high-quality sample preservation, as advancements in research and evolving healthcare requirements broaden use cases. The shift toward automated processes, improved monitoring systems, and tailored storage services addresses increasingly rigorous operational and compliance expectations, further driving market acceleration.Scope & Segmentation: Navigating the Biostorage Landscape
This report delivers senior leaders an actionable overview of the primary market segments shaping investment and operational decisions. Segments include:
- Offering: Differentiation between core equipment, comprehensive support services, and critical software functionalities tailored to storage management.
- Equipment Types: Coverage spans cryogenic storage, freezers, insulated containers, and specialized refrigeration for a range of preservation needs.
- Service Models: Emphasis on off-site storage facilities and value-added sample processing designed to streamline laboratory workflows.
- Software Features: Includes secure data management, real-time monitoring dashboards, and advanced analytics for decision support.
- Sample Types: Options for animal, human, microbial, and plant samples, enabling flexible research and commercial strategies.
- Storage Temperatures: Specifications address requirements from standard refrigeration to ultra-low and cryogenic environments, meeting diverse biological sample needs.
- Applications: Solutions for storing blood, microbial cultures, stem cells, tissues, and vaccines fit different research and diagnostic imperatives.
- End Users: Sectors served include academic institutions, biobank facilities, diagnostic laboratories, hospitals, and life science firms.
- Regions: Broad presence in the Americas, Europe, Middle East and Africa, and Asia-Pacific, with regional trends affecting technology uptake and regulatory priorities.
- Technologies: Integration of automation, modular and cloud-based storage, machine learning, and energy-efficient refrigeration supports both current and future needs.
- Sample Handling Practices: Strong focus on regulatory compliance, assurance of quality, and integrated digital solutions ensures consistency in operations.
Strategic developments by major players such as Thermo Fisher Scientific, Danaher Corporation, Bio-Rad Laboratories, Brooks Life Sciences, Cryoport, Haier Biomedical, and BioLife Solutions Inc. are examined throughout the analysis.
Key Takeaways for Decision-Makers
- Adoption of advanced storage technologies is intensifying due to the need for effective specimen tracking, quality assurance, and adherence to complex compliance frameworks.
- The convergence of automation, remote monitoring, and predictive analytics is streamlining operations while protecting the integrity of diverse biological samples.
- Collaborative approaches combining outsourced storage, processing, and data analytics support client retention and increase operational resilience.
- Regional variations in policy and funding drive the adoption of tailored solutions, with providers focused on compliance and sustainability achieving stronger positioning.
- Segment-specific requirements underscore the need for scalable, modular solutions capable of serving varied workflows and evolving project scopes.
- Growing sustainability priorities are influencing procurement of energy-efficient storage systems and the transition to digital, paperless recordkeeping initiatives.
Tariff Impact: Sector Resilience amid New U.S. Trade Policies
Recent U.S. tariffs on cryogenic and refrigeration imports are compelling biostorage manufacturers to adjust supply strategies, emphasizing domestic sourcing and local assembly. Distributors and service providers are revisiting contracts to accommodate cost increases. Executive responses include increasing purchase volumes, deploying predictive asset management, and establishing buffer inventories, reflecting an industry-wide adaptability to shifts in policy and cost structures while safeguarding operational reliability.
Research Methodology & Data Sources
The report employs a blend of secondary research from peer-reviewed literature, regulatory filings, patents, and manufacturer data, complemented by primary insights from interviews with laboratory and biobank professionals. Rigorous triangulation methods validate technological trends, operational priorities, and strategy shifts observed in the biostorage market.
The Biostorage Market: Why This Report Matters
- Provides C-level executives with a clear framework for technology assessment, investment alignment, and compliance planning in dynamic biostorage environments.
- Equips organizations with pragmatic recommendations to advance process efficiency, address regulatory objectives, and ensure supply chain resilience.
- Guides prioritization of investment and partnership opportunities through a segmented and actionable market overview.
Conclusion: Charting a Path Forward in Biostorage
Executives prioritizing modular solutions, compliance, and sustainability will strengthen their long-term position in biostorage. Strategic partnerships and adaptive leadership will be key for navigating changing demands and securing continued organizational growth.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Biostorage market report include:- Azenta US Inc.
- Binder GmbH
- Birka BioStorage AB
- Cryoport, Inc.
- Greiner AG
- Haier Biomedical
- Helmer Scientific Inc. Trane Technologies PLC
- Alcami Corporation
- MVE Biological Solutions
- PHC Holdings Corporation
- Planer Limited by Hamilton Thorne Inc
- Sartorius AG
- Simport Scientific Inc.
- Tescor by Link Group
- Thermo Fisher Scientific Inc.
- Versiti, Inc.
- Vigilant Bioservices
- X-Therma Inc.
- Precision Medicine Group, LLC
- ABS Bio, Inc.
- Precision Stability Storage
- IQVIA Inc.
- Cellutions Biostorage Pvt. Ltd.
- BioLife Solutions Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 199 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 4.75 Billion |
| Forecasted Market Value ( USD | $ 7.76 Billion |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |