Speak directly to the analyst to clarify any post sales queries you may have.
Unlocking the Future of Precision Infusion Therapy by Exploring Cutting-Edge Syringe Pump Technologies for Enhanced Patient Safety and Operational Efficiency
Healthcare providers worldwide are increasingly relying on precision infusion devices to improve treatment outcomes and enhance patient safety. Syringe pumps have become indispensable tools in critical care and ambulatory settings, delivering controlled doses of medications such as analgesics, antibiotics, and hormonal therapies. The ongoing shift towards minimally invasive procedures and personalized medicine further underscores the importance of highly accurate flow control mechanisms in clinical workflows. In this context, advancements in pump design, sensor integration, and materials engineering are driving significant performance improvements that meet the rigorous demands of modern healthcare systems.In addition, the expanding scope of home healthcare services has created new opportunities for remotely monitored infusion solutions that empower patients to manage complex therapies outside traditional hospital environments. This trend is supported by the growing adoption of telemedicine platforms and Internet of Things connectivity, enabling clinicians to track infusion parameters in real time and adjust therapy protocols as needed. Meanwhile, manufacturers are focusing on human-centered design principles to improve usability, reduce training requirements, and minimize the risk of medication errors across diverse care settings.
Overall, the syringe pump market is poised for transformative growth as the clinical community continues to prioritize precision, safety, and operational efficiency. Ongoing research into biocompatible materials, wireless communication protocols, and automated safety features promises to elevate the next generation of infusion devices. As stakeholders across the healthcare continuum collaborate to address emerging challenges such as drug shortages and regulatory complexity, syringe pump technology will remain at the forefront of therapeutic innovation.
Embracing Digital Transformation and IoT Integration to Drive the Next Wave of Innovation and Connectivity in the Global Syringe Pump Ecosystem
Over the past decade, digital transformation has reshaped the syringe pump landscape by introducing connected platforms that streamline device management and data analytics. Integration with hospital information systems and cloud-based solutions now enables remote monitoring of infusion parameters and predictive maintenance alerts. As a result, clinicians can proactively address anomalies, reduce downtime, and enhance patient safety through timely interventions.Simultaneously, the emergence of the Internet of Medical Things has opened pathways for real-time telemetry and advanced analytics. Devices equipped with smart sensors collect high-resolution flow and pressure metrics, empowering healthcare teams to fine-tune therapy regimens and anticipate device performance. These capabilities have accelerated the adoption of closed-loop infusion systems, marking a pivotal shift toward autonomous drug delivery that reduces manual oversight and variation in clinical outcomes.
Furthermore, material science breakthroughs have led to lighter, more resilient pump housings and disposable sets, while advances in battery technology extend operational runtimes for ambulatory and homecare applications. Collectively, these transformative shifts are driving a new era of syringe pump innovation characterized by agility, connectivity, and seamless integration into digital health ecosystems.
Assessing the Complex Impact of 2025 United States Tariff Adjustments on Supply Chain Dynamics and Cost Structures within the Syringe Pump Industry
The implementation of revised United States tariff schedules in 2025 has introduced multifaceted challenges for syringe pump manufacturers and their global supply networks. Manufacturers reliant on imported components have encountered increased input costs, prompting a strategic reassessment of sourcing practices. In response, several original equipment and contract manufacturers have initiated nearshoring strategies or diversified supplier portfolios to mitigate exposure to tariff-driven price pressures.These adjustments extend beyond procurement, influencing the entire production footprint. Extended lead times for critical raw materials such as stainless steel and specialized polymers have required more robust inventory management and safety stock protocols. At the same time, inflationary impacts on logistics and customs clearance have underscored the need for dynamic costing models that reflect evolving duty structures.
Amid these shifts, regulatory compliance and quality assurance have remained nonnegotiable demands. Industry stakeholders have accelerated technology transfer processes and intensified collaboration with certification bodies to ensure uninterrupted device validation. As stakeholders adapt to the new tariff regime, the emphasis on lean manufacturing techniques and digital traceability continues to grow, safeguarding both supply continuity and financial resilience.
Deep Diving into Market Segmentation Reveals Crucial Insights on Product Types Material Choices Modes of Operation and End User Distribution Trends
An in-depth examination of product variations reveals that infusion pump configurations dominate clinical usage due to their precision dosing capabilities, whereas withdrawal-specific designs cater to niche laboratory and research workflows requiring exact sample volumes. Material selection further differentiates the market, with glass constructs valued for chemical inertness in pharmaceutical manufacturing, plastic assemblies preferred for single-use applications in sterile environments, and stainless solutions chosen for their durability in high-throughput settings.The operational core of these systems spans electronic platforms offering programmable flow rates and pressure alarms to manual counterparts prized for simplicity in low-resource contexts. Electronic integration has garnered attention for its ability to generate adherence records and integrate with electronic health systems. Manual devices, however, remain relevant in settings demanding affordability and minimal technical support.
End-user environments also shape device specifications and adoption trends. Ambulatory surgical centers emphasize portability and user-friendly interfaces, while food and beverage laboratories focus on precision sample handling. Home healthcare providers require compact, quiet devices with long battery life for patient independence. Hospitals and clinics prioritize interoperability and scalability, pharmaceutical manufacturers demand compliance with stringent sterilization standards, and research institutes invest in high-resolution, programmable platforms for experimental reproducibility.
Regional Analysis Highlights Distinct Growth Drivers Regulatory Environments and Market Adoption Patterns across Americas Europe Middle East Africa and Asia Pacific
Across the Americas, broad healthcare infrastructure investments and emphasis on value-based care are propelling advanced infusion solutions into both urban hospitals and decentralized clinics. North American markets are particularly responsive to innovations in safety features, while Latin American providers seek cost-effective designs that balance performance with budget constraints. This regional landscape rewards adaptable partnerships that can localize production and support.In Europe, Middle East, and Africa, regulatory harmonization efforts and reimbursement frameworks are catalyzing market expansion. European nations with mature health systems prioritize interoperability and data security in connected pump installations. Middle Eastern markets are experiencing rapid adoption of next-generation devices thanks to significant capital infusions into healthcare modernization programs. Across Africa, initiatives addressing medication adherence and supply chain reliability are creating entry points for portable, robust devices that thrive in austere environments.
The Asia-Pacific region stands out for its dual emphasis on domestic manufacturing and digital health integration. High-growth economies are investing in local production hubs and forming strategic alliances to accelerate technology transfers. Simultaneously, the rise of home infusion therapies in densely populated markets fosters demand for compact, user-friendly devices that integrate with smartphone applications and telehealth services. This combination of industrial policy and consumer healthcare trends positions the Asia-Pacific landscape as a dynamic arena for syringe pump innovation.
Competitive Landscape Explored Through Strategic Profiles of Global Leading Industry Players Unveiling Innovation Portfolios Partnerships Operational Excellence and Positioning Dynamics
Leading industry participants are leveraging their legacy expertise and global footprints to secure competitive advantage. Baxter International has bolstered its portfolio through strategic acquisitions and a focus on intuitive user interfaces designed for high-volume infusion protocols. B. Braun has emphasized modular system architectures that allow seamless integration with hospital information platforms and supply management solutions.Terumo Corporation has invested heavily in next-generation sensor technologies and biocompatible materials, driving innovations in flow accuracy and patient safety. ICU Medical, renowned for its closed-system transfer devices, has expanded its infection control solutions to complement syringe pump offerings, addressing critical priorities in sterile drug delivery. Moog Inc. has prioritized custom engineering services for specialized clinical applications, fostering deep collaborations with research institutes to co-develop bespoke platforms for advanced therapeutic protocols.
Collectively, these players are intensifying R&D investments, forging partnerships to penetrate emerging markets, and refining aftermarket service models. Their combined efforts underscore a shift toward platform-based ecosystems that integrate hardware, software, and data analytics to deliver end-to-end infusion management solutions.
Action-Oriented Recommendations Empower Industry Leaders to Leverage Technological Innovation Streamline Operations Strengthen Market Resilience and Future-Proof Syringe Pump Manufacturing
Industry leaders must prioritize the integration of smart connectivity features that enable real-time performance monitoring and predictive maintenance. Investing in modular designs will allow rapid customization for diverse clinical and laboratory environments, enhancing product appeal and operational flexibility. Adopting agile manufacturing processes coupled with local assembly partnerships can mitigate geopolitical risks and tariff impacts while accelerating time-to-market.Furthermore, strengthening after-sales support networks through digital training platforms and remote diagnostics will reduce device downtime and elevate customer satisfaction. Collaborating with healthcare providers to co-create workflow-driven solutions will ensure seamless integration into existing clinical protocols and drive broader acceptance of advanced infusion technologies.
Finally, maintaining a proactive stance on regulatory alignment and quality management systems is essential. By embracing data-driven decision frameworks and fostering cross-functional teams that include regulatory experts, engineering talent, and clinical liaisons, organizations can navigate evolving standards efficiently and position themselves as trusted partners in the evolving syringe pump landscape.
Robust Research Methodology Outlines Comprehensive Systematic Data Collection Validation Techniques and Analytical Frameworks Ensuring Rigorous Insights into Syringe Pump Market Dynamics
Our research approach combines exhaustive secondary investigations with extensive primary engagements. We began by mapping the syringe pump ecosystem through a review of peer-reviewed journals, regulatory filings, patent databases, and industry white papers. This foundation informed targeted discussions with engineers, clinical end users, and supply chain executives to validate critical drivers and pain points.Data triangulation was conducted by cross-referencing insights from expert interviews against financial disclosures, regional procurement records, and device registration databases. Quantitative analyses leveraged statistical modeling techniques to identify correlations between design attributes, regional adoption rates, and operational efficiency metrics. Qualitative inputs were synthesized into thematic frameworks that capture technology trends, pricing dynamics, and strategic imperatives.
To ensure robustness, our methodology adheres to rigorous validation protocols, including peer reviews by independent subject matter experts and consistency checks across data sources. The result is a comprehensive, transparent, and reproducible body of insights that illuminates both current realities and future trajectories of the syringe pump market.
Conclusive Insights Summarize Emerging Opportunities Challenges and Strategic Imperatives Shaping the Transformative Future Trajectory of the Global Syringe Pump Sector
In conclusion, the syringe pump domain is undergoing a period of unparalleled innovation driven by digital connectivity, advanced materials, and shifting care delivery paradigms. The combined impact of regulatory changes, tariff realignments, and evolving end-user requirements underscores the need for adaptive strategies that address both operational efficiency and clinical performance.Key opportunities lie in the convergence of telehealth platforms with remotely monitored infusion systems, as well as in the development of eco-friendly consumables that meet stringent sustainability benchmarks. Strategic collaborations between device manufacturers and healthcare providers will be essential to co-create solutions that deliver measurable improvements in patient outcomes and cost containment.
By embracing a holistic perspective-encompassing segmentation insights, regional dynamics, and competitive positioning-stakeholders can navigate complexity and capitalize on growth vectors that redefine precision therapy. As the global healthcare ecosystem continues to evolve, syringe pumps will remain instrumental in enabling safe, effective, and patient-centric treatment modalities.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Infusion
- Withdrawal
- Material Type
- Glass
- Plastic
- Stainless
- Mode Of Operation
- Electronic
- Manual
- End User
- Ambulatory Surgical Centers
- Food & Beverage
- Home Healthcare
- Hospitals & Clinics
- Pharmaceutical
- Research Institutes
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Abbott Laboratories
- B. Braun Melsungen AG
- Baxter International Inc.
- Becton, Dickinson and Company
- Cavro Scientific Instruments by Tecan Group Ltd.
- Cetoni GmbH
- Chemyx, Inc.
- Cole-Parmer Instrument Company LLC
- Danaher Corporation
- Fresenius Kabi AG
- GE HealthCare Technologies, Inc.
- Hamilton & Company Limited.
- Harvard Apparatus Regenerative Technology, Inc
- ICU Medical, Inc.
- IMI plc
- Isco by Teledyne Technologies, Inc.,
- Medtronic PLC
- Merck KGaA
- Nihon Kohden Corporation
- Siemens Healthineers AG
- Terumo Corporation
- The New Era Pump Systems, Inc.
- World Precision Instruments GmbH
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Syringe Pumps market report include:- Abbott Laboratories
- B. Braun Melsungen AG
- Baxter International Inc.
- Becton, Dickinson and Company
- Cavro Scientific Instruments by Tecan Group Ltd.
- Cetoni GmbH
- Chemyx, Inc.
- Cole-Parmer Instrument Company LLC
- Danaher Corporation
- Fresenius Kabi AG
- GE HealthCare Technologies, Inc.
- Hamilton & Company Limited.
- Harvard Apparatus Regenerative Technology, Inc
- ICU Medical, Inc.
- IMI plc
- Isco by Teledyne Technologies, Inc.,
- Medtronic PLC
- Merck KGaA
- Nihon Kohden Corporation
- Siemens Healthineers AG
- Terumo Corporation
- The New Era Pump Systems, Inc.
- World Precision Instruments GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 197 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
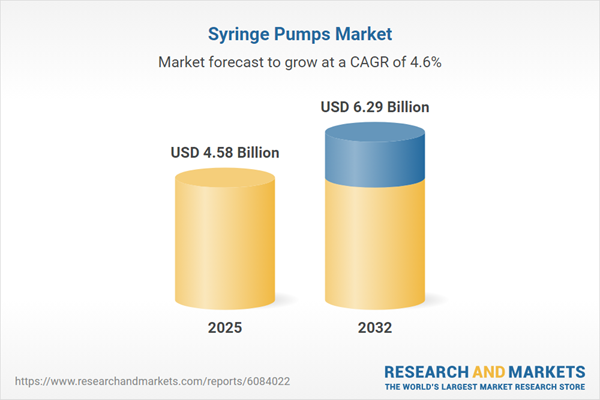
| Estimated Market Value ( USD | $ 4.58 Billion |
| Forecasted Market Value ( USD | $ 6.29 Billion |
| Compound Annual Growth Rate | 4.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |