Speak directly to the analyst to clarify any post sales queries you may have.
Unveiling Pivotal Developments Steering the Shift toward Disposable Nebulization Devices in Respiratory Care Environments
Unveiling the evolving landscape of respiratory care begins with an appreciation for the rising importance of single-use nebulization devices in both clinical and home settings. Escalating demand for accessible, hygienic inhalation therapies has been fueled by a growing prevalence of chronic respiratory conditions and heightened awareness of infection control. In parallel, technological progress has given rise to more efficient aerosol delivery systems that reduce treatment times and improve patient compliance.Moreover, healthcare providers and caregivers are increasingly seeking solutions that balance cost-effectiveness with reliability, driving adoption of disposables that eliminate cross-contamination risks without necessitating extensive maintenance protocols. This convergence of clinical necessity and operational efficiency underscores the strategic significance of disposable nebulization devices as a cornerstone of modern respiratory therapeutics. Looking ahead, the emphasis on patient-centric outcomes, regulatory rigor, and environmental considerations will continue to shape product innovation and value propositions across the continuum of care.
Examining Disruptive Innovations and Evolving Dynamics That Are Rapidly Redefining Disposable Nebulization Device Solutions
The disposable nebulization device field has undergone transformative change, propelled by disruptive innovations and shifting care models. Patient preferences for home administration have driven integration of user-friendly interfaces, compact form factors, and intuitive controls that facilitate independent use. Simultaneously, digital health integration has emerged as a game-changer, with connectivity features allowing remote monitoring of usage patterns and adherence metrics to inform clinical decision-making.Sustainability considerations have also catalyzed exploration of eco-friendly materials and end-of-life recycling pathways, prompting collaboration between device manufacturers and material science experts. Furthermore, the rise of personalized medicine has inspired advancements in device adaptability, enabling tailored aerosol particle sizes to address diverse patient anatomies and therapeutic regimens. Across hospital corridors and residential settings alike, these converging trends are redefining expectations for safety, convenience, and clinical efficacy in disposable nebulization technology.
Assessing the Comprehensive Implications of 2025 United States Import Tariffs on Supply Chain Dynamics and Cost Structures for Nebulization Devices
The introduction of new United States tariffs in 2025 has introduced significant considerations for supply chain resilience and cost structures within the disposable nebulization device domain. Manufacturers that have traditionally relied on imported components have faced elevated input costs, prompting reevaluation of sourcing strategies. This environment has accelerated the move toward local and regional manufacturing hubs to mitigate exposure to tariff volatility.In response, many stakeholders have streamlined component portfolios and engaged in strategic partnerships with domestic polymer and electronics suppliers. These collaborations aim to preserve product affordability while maintaining stringent performance standards. Regulatory compliance processes have also adapted, as procurement teams navigate evolving customs requirements and certification protocols. Despite these challenges, the tariff landscape has presented an opportunity for reshoring initiatives, fostering innovation in production efficiency and supply chain transparency that strengthen the long-term viability of disposable nebulization device offerings.
Unraveling Critical Segmentation Insights Across Product Types, Materials, Applications, End User Settings, and Distribution Channels for Strategic Positioning
Segment analysis reveals nuanced drivers that inform strategic positioning of disposable nebulization devices across diverse clinical and consumer use cases. In the realm of jet nebulizers, pneumatic devices remain prevalent in hospital and emergency medical settings due to their robustness and compatibility with existing compressed air systems, while electronic jet nebulizers are gaining traction within home healthcare for their portability and reduced noise levels. Mesh nebulizers, partitioned into static mesh and vibrating mesh subtypes, offer distinct advantages: static mesh models prioritize simplicity and cost efficiency, whereas vibrating mesh configurations deliver fine particle distribution essential for precision dosing in chronic obstructive pulmonary disease management.Ultrasonic nebulizers, classified by high-frequency and low-frequency operation, serve specialized applications; high-frequency units support rapid aerosolization in acute care scenarios, whereas low-frequency variants are favored for gentle aerosol delivery in pediatric and geriatric populations. Material composition further enhances product differentiation. Polycarbonate housings provide durability and optical clarity, polypropylene components balance cost and chemical resistance, while polyvinyl chloride and silicone elements optimize flexibility and patient comfort in mask or mouthpiece interfaces.
Applications such as asthma control, bronchitis relief, cystic fibrosis management, COPD therapy, and pulmonary infection treatment underline the device's versatility. End-user venues span ambulatory surgical centers seeking efficient short-term post-operative care solutions, clinics that demand reliable point-of-care devices, emergency medical services requiring ruggedized portables, home healthcare environments focused on ease of use, and hospitals that integrate disposables into infection-control protocols. Distribution channels encompass brick-and-mortar medical equipment stores and pharmacy outlets, alongside burgeoning online platforms that cater to direct-to-consumer fulfillment. Each segmentation pillar underscores distinct value propositions that align with evolving clinical workflows and patient expectations.
Highlighting Regional Variances and Growth Drivers Refining Adoption Patterns of Disposable Nebulization Devices Across Americas, EMEA, and Asia-Pacific
Regionally, adoption trajectories of disposable nebulization devices diverge according to healthcare infrastructure maturity, regulatory frameworks, and demographic trends. In the Americas, established reimbursement pathways and widespread home healthcare networks support robust utilization of compact, electronically enhanced nebulizers. Stakeholders in North and Latin America benefit from streamlined regulatory pathways that encourage swift product launches and iterative innovation.The Europe, Middle East & Africa region presents a mixed landscape: Western European markets uphold rigorous device certification standards and emphasize sustainability initiatives, driving interest in recyclable disposable components. Meanwhile, emerging economies within the Middle East and Africa are characterized by burgeoning demand for cost-effective, portable devices that address acute respiratory infection burdens and resource-constrained clinical settings.
Across Asia-Pacific, escalating prevalence of urban air pollution-related respiratory conditions, combined with rapidly expanding telehealth infrastructure, has fueled demand for connected, user-centric nebulization solutions. Regional manufacturing capabilities in East and Southeast Asia also bolster localized production of polymer and electronic subcomponents, offering competitive advantages in lead times and unit costs. These regional distinctions underscore the importance of tailoring product development and go-to-market strategies to align with localized regulatory, economic, and clinical care paradigms.
Illuminating Key Competitive Dynamics and Strategic Initiatives of Leading Organizations Shaping the Disposable Nebulization Device Landscape
Market leadership within the disposable nebulization device arena is shaped by established medical device corporations and agile specialized manufacturers. Prominent incumbents are advancing their portfolios through targeted research collaborations focused on optimizing aerosol delivery efficiency and integrating digital health monitoring. At the same time, niche innovators are capturing attention by pioneering biodegradable polymers and leveraging additive manufacturing to produce intricate mesh structures.Strategic alliances between device producers and pharmaceutical firms underscore a growing preference for combo therapies that pair specialized antibiotics or bronchodilators with dedicated delivery platforms. These collaborations aim to streamline therapy adherence and enhance clinical outcomes. Parallel efforts in clinical validation, including real-world evidence studies, are elevating the credibility of emerging offerings and informing reimbursement discussions.
Distribution partnerships are likewise evolving: leading device suppliers are forging exclusive agreements with global medical distributors that extend reach into underserved territories, while digital trade channels are enabling direct engagement with home healthcare providers and patient advocacy groups. Within this landscape, success hinges on balancing R&D intensity with market agility, ensuring product differentiation resonates with the unique needs of targeted end users.
Formulating Targeted Strategies and Operational Approaches to Seize Emerging Opportunities in Disposable Nebulization Devices
Industry stakeholders can capitalize on growth opportunities by embracing a multifaceted strategy that prioritizes innovation, supply chain diversification, and stakeholder collaboration. Focused investment in research and development of smart nebulization platforms-featuring real-time adherence tracking and remote care integration-can differentiate offerings within both clinical and at-home settings. Concurrently, establishing redundancy in component sourcing, including partnerships with regional suppliers, can shield operations from geopolitical and tariff-related disruptions.Engagement with regulatory bodies through early dialogue and participation in standards committees can streamline approval pathways and preempt compliance challenges. Moreover, alliances with patient advocacy organizations and home healthcare networks can yield insights into user preferences, guiding ergonomic design and user interface enhancements. Operationally, adopting lean manufacturing principles and modular assembly approaches can reduce production lead times and improve cost efficiency. Collectively, these recommendations empower decision-makers to navigate evolving care paradigms and capture incremental value across the disposable nebulization device continuum.
Detailing the Robust Mixed-Methods Approach, Stakeholder Engagement, and Analytical Techniques Applied to Nebulization Device Insights
This research employs a rigorous mixed-methods framework that integrates primary stakeholder engagement with comprehensive analysis of secondary literature. Primary inputs were obtained through structured interviews with clinicians, procurement specialists, and device engineers spanning acute care facilities, home healthcare providers, and emergency medical services. These perspectives were triangulated with internal technical dossiers, regulatory filings, and peer-reviewed clinical studies to validate device performance attributes and adoption drivers.Secondary research encompassed examination of industry white papers, medical association guidelines, and polymer science publications to contextualize material innovations and device durability. Supply chain dynamics were assessed through trade data reviews and consultations with logistics experts to understand tariff impacts and regional manufacturing capabilities. Qualitative insights were synthesized via thematic analysis, while quantitative data on device utilization patterns and cost factors were processed through statistical benchmarking. This methodological approach ensures that conclusions are grounded in robust evidence and diverse expert viewpoints.
Synthesizing Core Insights and Strategic Imperatives to Navigate the Future Evolution of Disposable Nebulization Device Solutions
In synthesizing these findings, the narrative underscores the confluence of technological innovation, regulatory evolution, and shifting care delivery models that define the current trajectory of disposable nebulization devices. From segmentation nuances in device architecture and material composition to regional adoption disparities and tariff-driven supply chain recalibrations, each dimension reveals distinct imperatives for product development and market positioning.The competitive landscape is marked by collaborative research initiatives, strategic distribution alliances, and a growing emphasis on digital health integration. As patient expectations and clinical protocols evolve, stakeholders that align device differentiation with end-user requirements and operational efficiencies will secure the greatest advantage. Ultimately, an evidence-based, agile approach to R&D, manufacturing, and go-to-market planning will be essential for navigating the complexities of a rapidly transforming respiratory care environment.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Jet Nebulizers
- Electronic Devices
- Pneumatic Devices
- Mesh Nebulizers
- Static Mesh
- Vibrating Mesh
- Ultrasonic Nebulizers
- High Frequency
- Low Frequency
- Jet Nebulizers
- Material
- Polycarbonate (PC)
- Polypropylene (PP)
- Polyvinyl Chloride (PVC)
- Silicone
- Application
- Asthma
- Bronchitis
- Chronic Obstructive Pulmonary Disease (COPD)
- Cystic Fibrosis
- Pulmonary Infections
- End User
- Ambulatory Surgical Centers
- Clinics
- Emergency Medical Services (EMS)
- Home Healthcare Settings
- Hospitals
- Distribution Channel
- Offline Channel
- Medical Equipment Stores
- Pharmacies
- Online Channel
- Offline Channel
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Angiplast Pvt. Ltd.
- Cardinal Health, Inc.
- DeVilbiss Healthcare, LLC
- Hamilton Medical AG
- Intersurgical Limited
- Koninklijke Philips N.V.
- Lepu Medical Technology (Beijing) Co., Ltd.
- Medline Industries, LP.
- Omron Corporation
- PARI GmbH
- Salter Laboratories, Inc.
- SunMed Group Holdings, LLC dba AirLife
- Apex Medical Co., Ltd.
- GF Health Products, Inc.
- Allied Healthcare Products, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Disposable Nebulizition Device market report include:- Angiplast Pvt. Ltd.
- Cardinal Health, Inc.
- DeVilbiss Healthcare, LLC
- Hamilton Medical AG
- Intersurgical Limited
- Koninklijke Philips N.V.
- Lepu Medical Technology (Beijing) Co., Ltd.
- Medline Industries, LP.
- Omron Corporation
- PARI GmbH
- Salter Laboratories, Inc.
- SunMed Group Holdings, LLC dba AirLife
- Apex Medical Co., Ltd.
- GF Health Products, Inc.
- Allied Healthcare Products, Inc.
Table Information
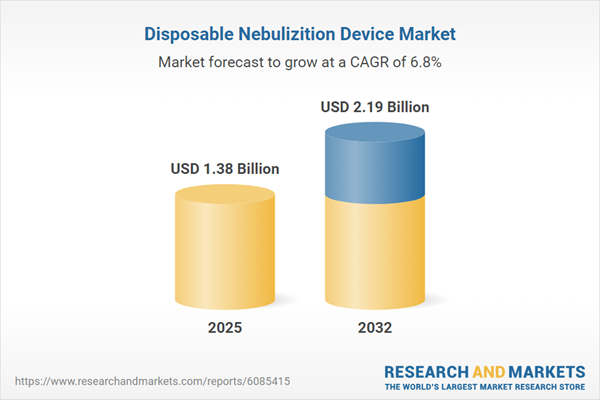
| Report Attribute | Details |
|---|---|
| No. of Pages | 198 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 1.38 Billion |
| Forecasted Market Value ( USD | $ 2.19 Billion |
| Compound Annual Growth Rate | 6.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |