Speak directly to the analyst to clarify any post sales queries you may have.
Setting the Stage for Sterile Gauze Dressing Phenomenon: Critical Context on Innovations, Demand Drivers, and Healthcare Imperatives
To begin with, the sterile gauze dressing sector has emerged as a cornerstone in advanced wound care and surgical management, underpinning outcomes across acute, chronic, and trauma-related treatment settings. Growing clinical complexity, coupled with the imperative to reduce healthcare-associated infections, has propelled healthcare providers to reexamine the performance attributes, material compositions, and delivery systems of sterile gauze products. Moreover, demographic shifts and increasing prevalence of comorbidities have intensified demand for versatile dressing solutions that can adapt to diverse wound etiologies and patient needs.Against this backdrop, escalating emphasis on cost containment and value-based care is reshaping procurement frameworks, compelling manufacturers and distributors to innovate across the value chain. From raw material selection through sterilization and regulatory compliance, every stage of the supply pathway is under scrutiny. Consequently, understanding the interplay between clinical outcomes, regulatory landscapes, and cost drivers becomes critical for stakeholders seeking to navigate the evolving sterile gauze environment.
As such, this analysis establishes foundational context by examining the core drivers shaping sterile gauze adoption, including infection control imperatives, performance benchmarks, and emerging care delivery models. It sets the stage for an in-depth exploration of market shifts, policy impacts, segmentation dynamics, and strategic imperatives that will define the competitive landscape over the coming years.
Navigating Rapid Transformations in Sterile Gauze Market Dynamics as Technological Breakthroughs and Patient-Centric Care Reshape Clinical and Surgical Practices
In recent years, the sterile gauze domain has witnessed a profound metamorphosis driven by technological breakthroughs and a relentless pivot toward patient-centric care. Enhanced fiber engineering techniques have yielded hydrophilic and antimicrobial gauze substrates that accelerate wound healing while minimizing bioburden. Simultaneously, the integration of sensor-embedded dressings and digital monitoring systems is enabling real-time tracking of exudate levels and pH fluctuations, fostering data-driven clinical decisions and remote care capabilities.Alongside material and digital innovations, shifting care protocols are redefining usage patterns. The move from inpatient to outpatient and home-based wound management has intensified demand for ready-to-use, easy-apply formats that empower patients and caregivers. Sustainability priorities are further catalyzing the adoption of recyclable packaging formats and bio-derived fibers, as healthcare institutions seek to minimize environmental footprints without compromising safety standards.
Collectively, these paradigm shifts are realigning stakeholder expectations around performance, convenience, and environmental stewardship. As stakeholders embrace these disruptive trends, the sterile gauze landscape is becoming more dynamic, requiring agile strategies that anticipate evolving clinical imperatives and value frameworks.
Unveiling the Far-Reaching Effects of 2025 Tariff Measures on Cross-Border Trade Flows, Production Costs, and Strategic Sourcing of Sterile Gauze Dressings
The introduction of new tariff measures in 2025 has introduced a layer of complexity to the global sterile gauze supply chain, exerting pressure on raw material costs and cross-border procurement strategies. Heightened duties on imported cotton fibers and specialized nonwoven substrates have amplified production expenditures for manufacturers relying on offshore suppliers. This cost increase is reverberating across the value chain, prompting contract renegotiations and localized sourcing initiatives to mitigate import exposure.Consequently, some manufacturers are accelerating the diversification of their supply bases, forging partnerships with domestic producers of spunlace and woven cotton gauze. Others are exploring vertical integration to internalize critical processes such as sterilization and fabric treatment. As organizations adapt, strategic buyers are reassessing lead times and inventory buffers, balancing cost optimization against the risk of supply disruptions.
Ultimately, the cumulative impact of these tariff developments underscores the need for proactive supply chain transparency and scenario planning. Organizations that invest in robust procurement analytics and cultivate resilient supplier ecosystems will be best positioned to absorb cost fluctuations while maintaining reliable access to high-quality sterile gauze products.
Unraveling Critical Insights Across Multifaceted Sterile Gauze Segmentation to Illuminate Product, Material, Packaging, Application, and Distribution Trends
A nuanced understanding of how different sterile gauze formats perform across clinical settings is essential for tailoring solutions to patient and institutional requirements. Within the broad category of product configurations, pads, rolls, and sponges each exhibit unique absorbency profiles, conformability characteristics, and dressing change frequencies that influence clinical workflows. Pads excel in covering flat or minimally contoured wounds, rolls offer customizable lengths for larger or irregular sites, and sponges deliver enhanced cushioning for cavity or tunneling wounds.Material selection further differentiates sterile gauze offerings. Cotton gauze retains its position as the trusted standard for its natural absorbency and compatibility with topical agents, whereas polyester gauze provides a synthetic alternative with superior tensile strength and reduced fiber shed. This material dichotomy shapes performance benchmarks in diverse applications, from high-exudate chronic wounds to delicate surgical incision sites.
Packaging methods also drive user preferences and operational efficiency. Bulk packaging optimizes inventory management and reduces per-unit costs in high-volume hospital systems, while individual packaging enhances sterility assurance and point-of-care convenience in ambulatory surgical centers and home care settings. The choice between these models involves trade-offs in waste management, storage requirements, and application speed.
Clinical purpose remains a critical segmentation dimension for sterile gauze application. Burn injuries demand high-capacity exudate removal and infection control adjuncts, chronic wounds necessitate extended wear times with moisture balance, surgical wounds require low-lint surfaces and precise sizing, and trauma wounds benefit from rapid hemostasis and conformable sealing. This application diversity underpins differentiated product development and targeted commercialization strategies.
End users encompass a spectrum of care settings, each with distinct purchasing protocols and clinical protocols. Ambulatory surgical centers prioritize streamlined procedural workflows and cost controls, home healthcare providers focus on ease of application and patient self-management, and hospitals and clinics demand supply chain robustness and regulatory compliance. These end-user imperatives drive portfolio configurations and service offerings.
Distribution channels are evolving in parallel. Traditional offline pathways remain vital for institutions with centralized procurement systems, while online channels are gaining prominence among smaller facilities and direct-to-consumer models. Within the online sphere, e-commerce platforms offer broad product assortments and competitive pricing, whereas manufacturer websites enable direct engagement, customized ordering, and bundled service packages tailored to enterprise clients.
Decoding Regional Variations in Sterile Gauze Adoption Across the Americas, Europe Middle East & Africa, and Asia-Pacific Therapeutic Environments
Regional dynamics are shaping the sterile gauze landscape in ways that reflect healthcare infrastructure maturity, regulatory frameworks, and procurement modalities. In the Americas, well-established hospital networks and a growing focus on outpatient and home care have elevated demand for user-friendly dressing formats and integrated supply solutions. North American providers, in particular, are investing in digital inventory management systems to streamline reordering and reduce waste, while Latin American markets are witnessing increased public sector initiatives to bolster access to essential wound care products.Across Europe, the Middle East, and Africa, regulatory harmonization and public health campaigns aimed at reducing wound-related complications are steering sterilization standards and product approval pathways. Western European healthcare systems emphasize strict biocompatibility testing and environmental sustainability criteria, prompting manufacturers to adopt eco-friendly materials and closed-loop packaging systems. In the Middle East and Africa, expanding private hospital networks and growing trauma care capabilities are driving investments in high-performance gauze dressings tailored to acute injury management.
The Asia-Pacific region is experiencing rapid expansion of local manufacturing capacity, leveraging lower production costs and government incentives to serve both domestic and export markets. At the same time, rising healthcare access in emerging economies is fueling demand for cost-effective yet clinically robust sterile gauze solutions. In advanced Asia-Pacific economies, emphasis on precision medicine and smart dressings is fostering early adoption of sensor-enabled gauze formats and integrated telehealth offerings.
Profiling Leading Sterile Gauze Manufacturers: Competitive Strategies, Innovation Portfolios, and Collaborative Networks Driving Market Leadership
Leading participants in the sterile gauze arena are advancing competitive differentiation through strategic product innovation, targeted partnerships, and operational excellence. A multinational healthcare conglomerate has recently expanded its gauze portfolio with fiber-blend substrates impregnated with broad-spectrum antimicrobial compounds, forging alliances with research institutions to validate clinical efficacy. Another major player has optimized its global manufacturing footprint through a series of capacity additions in geographically strategic locations, improving lead times and cost competitiveness.Notable mid-tier companies are leveraging digital channels to enhance customer engagement, deploying online platforms that integrate ordering, inventory tracking, and value-added educational resources. Collaborative networks between manufacturers and distribution partners have intensified, as stakeholders seek to align on demand forecasting methodologies, consignment models, and just-in-time delivery frameworks. These alliances are redefining service paradigms, moving beyond product supply toward outcome-based contracting.
Smaller specialized firms are carving out niches by offering customizable gauze elements, such as perforated or pre-cut shapes, as well as co-developed solutions for complex wound types. Through selective acquisitions and licensing arrangements, these innovators are scaling their technologies while preserving agility and customer intimacy. Collectively, these competitive maneuvers illustrate a market in which continuous innovation and strategic collaboration are key drivers of leadership.
Empowering Industry Stakeholders with Actionable Strategies to Navigate Regulatory Complexities, Optimize Supply Chains, and Accelerate Value Creation in Sterile Gauze
Industry stakeholders should prioritize engagement with regulatory authorities to anticipate shifts in sterilization guidelines and environmental mandates, ensuring product pipelines remain compliant and market-ready. Investing in advanced fiber technologies that combine absorbency with antimicrobial protection can create differentiated value propositions, especially when validated by real-world clinical evidence. Moreover, integrating digital monitoring capabilities within gauze substrates offers a pathway to elevate patient outcomes while generating actionable data for care teams.Supply chain resilience must be reinforced through diversified sourcing strategies and strategic inventory buffers. Establishing partnerships with regional fiber producers and contract sterilizers can mitigate exposure to tariff-related cost increases and transportation delays. In parallel, adopting predictive analytics for demand planning and dynamic reorder thresholds will help maintain service levels without excess carrying costs.
From a commercial perspective, organizations should develop tailored go-to-market approaches that address the distinct needs of ambulatory surgical centers, home care providers, and hospital systems. Crafting value-based agreements that link product performance to reimbursement incentives can foster deeper customer relationships. Finally, sustainability imperatives present an opportunity to implement closed-loop packaging reuse programs and bio-based material initiatives, reinforcing brand reputation and regulatory alignment.
Elucidating Rigorous Research Methodology Employing Primary Interactions, Secondary Analysis, and Robust Validation to Ensure High-Integrity Sterile Gauze Insights
This analysis is grounded in a hybrid research framework that synthesizes primary interactions with senior executives across manufacturing, distribution, and clinical procurement functions, and a comprehensive review of secondary sources, including peer-reviewed journals, regulatory filings, and specialized industry publications. Primary engagements involved in-depth interviews to harvest qualitative insights on innovation roadmaps, tariff adaptation strategies, and end-user preferences.Secondary research served to validate trends through triangulation with public data, including regulatory announcements and patent filings. A rigorous data-validation process was employed to reconcile discrepancies between reported production volumes, import/export statistics, and firsthand practitioner feedback. Furthermore, supplemental consultations with clinical experts and supply chain specialists ensured that the analysis accurately reflects operational realities and emerging best practices.
Together, these methodological layers provide a robust foundation for actionable conclusions, enabling stakeholders to trust the integrity of the insights and apply them directly to strategic decision-making processes.
Summarizing Core Findings and Strategic Imperatives to Equip Decision-Makers with a Consolidated Perspective on the Sterile Gauze Dressing Sector
In summary, the sterile gauze sector stands at an inflection point defined by material innovation, digital integration, and evolving procurement frameworks. Technological advancements are expanding the functional capabilities of gauze products, while regulatory and tariff dynamics are reshaping supply chain architectures. A detailed segmentation analysis underscores the importance of aligning product attributes with clinical applications and end-user workflows, and regional perspectives highlight the heterogeneous drivers across key geographies.Competitive positioning will increasingly hinge on the ability to deliver differentiated solutions that balance performance, cost, and sustainability imperatives. Stakeholders equipped with a clear understanding of segmentation dynamics, tariff impacts, and strategic company profiles will be better prepared to capitalize on emerging opportunities. As healthcare delivery models continue to evolve, proactive adaptation and collaboration will be essential for sustained leadership in the sterile gauze domain.
This consolidated perspective offers decision-makers a roadmap to navigate complexity, unlock innovation potential, and drive meaningful advancements in wound care outcomes.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Sterile Gauze Pads
- Sterile Gauze Rolls
- Sterile Gauze Sponges
- Material Type
- Cotton Gauze
- Polyester Gauze
- Packaging Type
- Bulk Packaging
- Individual Packaging
- Application
- Burns
- Chronic Wounds
- Surgical Wounds
- Trauma Wounds
- End User
- Ambulatory Surgical Centers
- Home Healthcare
- Hospitals & Clinics
- Distribution Channel
- Offline
- Online
- E-commerce Platforms
- Manufacturer Websites
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- 3M Company
- B. Braun SE
- Cardinal Health, Inc.
- Covidien PLC
- DermaRite Industries, LLC
- Dukal Corporation
- Dynarex Corporation
- Essity AB
- GFA Production Xiamen Co., Ltd
- Johnson & Johnson Services, Inc.
- Lohmann & Rauscher GmbH & Co. KG
- McKesson Medical-Surgical Inc.
- Medline Industries, LP
- Mölnlycke Health Care AB
- NDC, Inc.
- Paul Hartmann AG
- SDP Inc.
- Smith & Nephew PLC
- Texpol S.A.
- TIDI Products, LLC
- Winner Medical Co., Ltd.
- Yangzhou VIOMED
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Sterile Gauze Dressing market report include:- 3M Company
- B. Braun SE
- Cardinal Health, Inc.
- Covidien PLC
- DermaRite Industries, LLC
- Dukal Corporation
- Dynarex Corporation
- Essity AB
- GFA Production Xiamen Co., Ltd
- Johnson & Johnson Services, Inc.
- Lohmann & Rauscher GmbH & Co. KG
- McKesson Medical-Surgical Inc.
- Medline Industries, LP
- Mölnlycke Health Care AB
- NDC, Inc.
- Paul Hartmann AG
- SDP Inc.
- Smith & Nephew PLC
- Texpol S.A.
- TIDI Products, LLC
- Winner Medical Co., Ltd.
- Yangzhou VIOMED
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
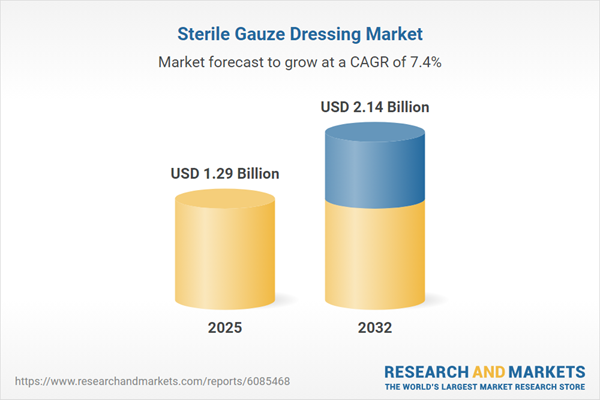
| Estimated Market Value ( USD | $ 1.29 Billion |
| Forecasted Market Value ( USD | $ 2.14 Billion |
| Compound Annual Growth Rate | 7.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |