Speak directly to the analyst to clarify any post sales queries you may have.
Exploring the transformative potential and foundational advancements that define small nucleic acid therapies within modern precision medicine landscapes
Small nucleic acid therapies have emerged as a foundational pillar in the evolution of modern therapeutics, offering unprecedented precision in targeting genetic and molecular pathways that underlie a wide array of diseases. These modalities encompass a diverse set of molecules including antisense oligonucleotides, aptamers, microRNA therapeutics, messenger RNA constructs, piwi-interacting RNAs, and small interfering RNAs. By binding to specific RNA sequences or modulating gene expression, they can correct pathogenic mechanisms at their source rather than merely alleviating symptoms.This introduction highlights the catalytic role of these therapies in reshaping treatment paradigms across multiple therapeutic areas. Antisense oligonucleotides, for instance, leverage both RNase H-mediated degradation and steric-blocking mechanisms to silence or modulate target transcripts with clinical success stories such as inotersen and nusinersen. Similarly, emerging platforms such as lipid nanoparticle-delivered mRNA have proven their value in vaccine development and beyond. Consequently, the synergy between scientific innovation, enabling delivery technologies, and regulatory support has accelerated the translation from bench to bedside.
As the landscape continues to mature, stakeholders must recognize the foundational advancements that have paved the way. This overview sets the stage for a deeper examination of transformative shifts, regulatory dynamics, segmentation insights, regional performance, industry leadership, recommended actions, methodological rigor, and a concluding synthesis of strategic imperatives.
Identifying the pivotal scientific breakthroughs, regulatory milestones, and strategic collaborations reshaping the small nucleic acid therapy ecosystem
Over the past decade, a series of scientific breakthroughs and regulatory milestones have converged to transform the small nucleic acid therapy landscape. Initially constrained by delivery challenges and concerns over off-target effects, these modalities have benefited from advances in delivery vehicles such as GalNAc conjugation and lipid nanoparticles, which have significantly improved cellular uptake and tissue specificity. Moreover, polymer-based carriers and engineered viral vectors have expanded the repertoire of organ targets beyond the liver, enabling renewed focus on neurological and oncology indications.Regulatory bodies around the world have also played an instrumental role in catalyzing this evolution. Historic approvals of antisense oligonucleotide drugs demonstrated clear clinical benefits in rare genetic disorders, underlining the feasibility of these approaches. As a result, accelerated pathways and adaptive licensing frameworks have fostered greater confidence among researchers and investors, encouraging the launch of novel platforms and combination strategies.
In parallel, strategic collaborations between academic institutions, biotech innovators, and large pharmaceutical companies have expanded the scope of translational research. Consequently, the pace of early-stage pipeline development has increased, ushering in a more diverse and robust array of candidates across multiple molecular classes. Taken together, these cumulative developments underscore a dynamic environment in which small nucleic acid therapies are no longer niche interventions but central components of the precision medicine toolkit.
Evaluating the ripple effects of 2025 U.S. tariffs on raw materials, import logistics, and supply chain stability in the small nucleic acid therapies industry
In 2025, the introduction of new U.S. tariff measures on imported raw materials and specialized reagents has had a profound impact on the small nucleic acid therapy supply chain. Many critical components, such as nucleotide analogs, delivery vehicle precursors, and certain laboratory consumables, are sourced internationally. Tariff-induced cost increases have compelled manufacturers to reassess procurement strategies and explore alternative supplier networks. Consequently, some organizations have accelerated nearshoring initiatives to mitigate exposure to import duties and potential geopolitical fluctuations.Furthermore, the additional financial burden associated with device imports, including specialized bioreactors and automation systems, has extended lead times for production scale-up. As a result of these logistical adjustments, development timelines have elongated, prompting project teams to incorporate contingency buffers. At the same time, increased coordination with customs authorities and third-party logistics providers has become essential to maintain continuity of critical raw material flows.
Despite these challenges, proactive companies have begun to adopt comprehensive risk-management frameworks that emphasize supplier diversification and inventory optimization. In addition, collaborative efforts between industry consortia and regulatory agencies are exploring mechanisms to streamline tariff classification and facilitate expedited clearance processes. Through these adaptive strategies, stakeholders aim to preserve the momentum of therapeutic innovation, ensuring that tariff pressures do not impede the delivery of life-changing interventions to patients.
Revealing pivotal segmentation insights across therapy types, administration routes, delivery platforms, therapeutic areas, and end user categories
Segmentation analysis reveals that therapy type distinctions drive differentiated development strategies across the small nucleic acid ecosystem. Antisense oligonucleotides are further categorized by RNase H-dependent mechanisms exemplified by agents such as fomivirsen, inotersen, and mipomersen, alongside steric-blocker oligonucleotides represented by eteplirsen, golodirsen, and nusinersen. Aptamer platforms are likewise bifurcated into DNA and RNA configurations, each offering unique affinities and stability profiles. MicroRNA and piwi-interacting RNA therapeutics have shown promise in fine-tuning gene networks, whereas messenger RNA constructs have gained prominence through vaccine applications. Small interfering RNAs, led by pioneering candidates like givosiran and patisiran, continue to expand into metabolic and rare disease domains.Based on route of administration, the market spans oral delivery solutions as well as parenteral options, with intramuscular, intravenous, and subcutaneous injections tailored to diverse clinical needs. This dynamic interplay between administration modalities and therapeutic targets necessitates careful consideration of pharmacokinetics and patient compliance.
Delivery mechanisms constitute a pivotal dimension of market segmentation, encompassing targeted GalNAc conjugation for hepatic indications, lipid nanoparticle formulations capable of broad organ distribution, polymer-based carriers engineered for controlled release, and viral vectors designed for precise cellular transduction.
Therapeutic applications extend across cardiovascular, genetic, infectious, inflammatory, neurological, oncological, and ophthalmic disease areas. Within genetic disorders, rare conditions such as Duchenne muscular dystrophy and spinal muscular atrophy underscore the potential for disease-modifying outcomes. Infectious disease efforts target both bacterial and viral pathogens, while inflammatory and autoimmune segments address psoriasis and rheumatoid arthritis. Neurological indications such as Alzheimer's and Parkinson's disease are under active investigation, and oncology pipelines feature challenging targets like glioblastoma and hepatocellular carcinoma.
From an end user perspective, academic and research institutes continue to spearhead fundamental discoveries, supported by contract research organizations that enable efficient preclinical evaluation. Hospitals and specialty clinics drive translational applications, and pharmaceutical and biotechnology companies lead commercial development and distribution.
Examining regional dynamics shaping the small nucleic acid therapies sector across the Americas, Europe Middle East & Africa, and Asia-Pacific landscapes
Regional dynamics play a critical role in shaping the small nucleic acid therapy sector, with each geography exhibiting distinct enablers and barriers. In the Americas, a well-established regulatory framework and robust funding ecosystem have accelerated clinical trial initiation and facilitated partnerships between academic centers and industry sponsors. This environment has historically produced landmark approvals and a thriving startup community.Europe, the Middle East & Africa present a multifaceted landscape in which varying regulatory harmonization efforts and reimbursement policies influence market adoption. Countries within the European Union benefit from centralized review processes, while Middle Eastern and African markets are navigating emerging frameworks that prioritize access to innovative therapies. Collaborative initiatives among regional consortia aim to standardize clinical requirements and reduce time-to-market across multiple jurisdictions.
Asia-Pacific markets demonstrate dynamic growth driven by large patient populations, increasing healthcare expenditure, and government-led precision medicine initiatives. Regional leaders are prioritizing the development of local manufacturing infrastructure to decrease reliance on imports and support domestic innovation. These investments, combined with streamlined approval pathways in select markets, are fostering an increasingly competitive and diversified therapeutic landscape.
Taken together, these regional characteristics underscore the importance of tailored strategies. Companies seeking global reach must adapt to shifting policy environments, establish local partnerships, and engage with key stakeholders to ensure sustainable access and adoption.
Highlighting leading innovators and strategic partnerships among pharmaceutical and biotech companies driving advancements in small nucleic acid therapies
Leading companies in the small nucleic acid therapy arena have distinguished themselves through strategic platform investments, research collaborations, and targeted pipeline portfolios. Innovators pioneering antisense oligonucleotide technologies have demonstrated the clinical viability of gene silencing in rare diseases, while mRNA developers have showcased rapid response capabilities in vaccine and therapeutic domains. Entities specializing in siRNA have built a foundation of experience in hepatic targeting, evolving toward indications that require sophisticated delivery platforms.In addition to proprietary research, many organizations have pursued partnerships to access emerging modalities and expand their technical expertise. For example, collaborations between established pharmaceutical firms and biotech startups have enabled the integration of novel conjugation chemistries and advanced carrier systems. Consequently, these alliances have accelerated milestone achievements and diversified candidate profiles.
Investment trends further reflect a balance between internal discovery programs and external innovation through licensing and joint ventures. Venture capital and strategic corporate funding continue to support early-stage companies, while larger players leverage their global infrastructures to scale manufacturing and commercial operations. The combined focus on translational research, bench-to-clinic studies, and supply chain optimization exemplifies a cohesive industry approach to overcoming development hurdles.
As competitive dynamics evolve, companies that maintain agility in platform adaptation, cultivate regulatory expertise, and engage stakeholders across the value chain will remain positioned at the forefront of therapeutic innovation.
Formulating actionable recommendations for industry leaders to enhance innovation, fortify supply chains, and expedite development of nucleic acid therapies
Industry leaders must adopt a proactive stance to capitalize on the burgeoning opportunities in nucleic acid therapeutics. First, enhancing research and development pipelines through strategic platform diversification can mitigate technical risks and broaden indication portfolios. By integrating complementary delivery mechanisms and exploring next-generation conjugation strategies, organizations can improve target specificity and clinical efficacy.Second, reinforcing supply chain resilience is essential to maintaining uninterrupted development and commercialization timelines. This can be achieved by cultivating relationships with multiple raw material suppliers, establishing regional manufacturing capabilities, and implementing real-time inventory monitoring systems. In doing so, companies can buffer against tariff fluctuations and logistical disruptions.
Furthermore, forging collaborative partnerships with regulatory authorities and academic institutions can streamline clinical trial design and expedite approval processes. Engaging in early dialogue on trial endpoints and safety criteria enables more efficient study execution and can unlock accelerated pathways for high-unmet-need indications.
Finally, investing in data analytics and digital health integration will support patient engagement and post-market surveillance. Leveraging real-world evidence platforms and connected devices enhances outcome tracking and informs iterative improvements to therapy regimens. Collectively, these initiatives will drive sustainable growth and ensure that nucleic acid therapeutics fulfill their transformative potential.
Detailing the comprehensive research methodology, data collection techniques, and analytical frameworks underpinning the small nucleic acid therapy market study
The research methodology underpinning this executive summary combines rigorous secondary research with targeted primary insights to ensure comprehensive and accurate analysis. Initially, extensive literature reviews were conducted across scientific journals, patent filings, regulatory archives, and corporate disclosures to map the historical evolution of nucleic acid therapy platforms. This phase provided foundational context for technology trajectories and competitive landscapes.Subsequently, expert interviews with key opinion leaders, industry executives, and clinical researchers enriched the data set with firsthand perspectives on development challenges, patient population dynamics, and regulatory considerations. These qualitative inputs were systematically validated against publicly available trial registries and financial disclosures to confirm consistency and relevance.
Analytical frameworks employed include SWOT analyses at the modality and company levels, supply chain impact assessments, and segmentation mapping across therapeutic applications and regional markets. Cross-validation techniques were applied to reconcile differences between primary insights and literature-based findings, thereby enhancing reliability. Data synthesis followed an iterative process, with regular alignment checks to ensure coherence and to identify emerging trends.
By integrating multiple research vectors-document analysis, expert consultation, and quantitative cross-validation-this methodology delivers a robust foundation for strategic decision-making and actionable recommendations.
Synthesizing key findings and strategic imperatives to underscore the future trajectory and potential of small nucleic acid therapies in precision healthcare
In conclusion, the maturation of small nucleic acid therapies heralds a new era in precision medicine, where molecular interventions target disease at its genetic and biochemical roots. The confluence of advanced delivery technologies, favorable regulatory frameworks, and strategic collaborations has transformed early-stage concepts into approved treatments and robust pipelines. Furthermore, emerging tariff dynamics and regional policy shifts underscore the need for adaptive strategies that can safeguard supply chain continuity and market access.Key segmentation insights reveal diverse modality profiles, administration preferences, and therapeutic priorities that must be addressed through tailored development plans. Regional analysis highlights the imperative to navigate disparate regulatory environments while leveraging local manufacturing and clinical expertise. Leading companies have demonstrated the value of platform integration, alliance formation, and investment discipline, setting benchmarks for innovation and execution.
Looking ahead, stakeholders who proactively diversify their technology portfolios, strengthen operational resilience, and engage in collaborative regulatory dialogue will be best positioned to harness the full potential of nucleic acid therapies. Through data-driven decision-making and patient-centric development approaches, this sector is poised to deliver unprecedented therapeutic benefits across a spectrum of diseases.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Therapy Type
- Antisense Oligonucleotides
- RNase H-dependent Oligonucleotides
- Fomivirsen
- Inotersen
- Mipomersen
- Steric-Blocker Oligonucleotides
- Eteplirsen
- Golodirsen
- Nusinersen
- RNase H-dependent Oligonucleotides
- Aptamers
- DNA Aptamers
- RNA Aptamers
- miRNA Therapeutics
- mRNA Therapeutics
- piRNA Therapeutics
- siRNA Therapeutics
- Givosiran
- Patisiran
- Antisense Oligonucleotides
- Route of Administration
- Oral Route
- Parenteral Route
- Intramuscular
- Intravenous
- Subcutaneous
- Delivery Mechanism
- GalNAc Conjugation
- Lipid Nanoparticles (LNPs)
- Polymer-Based Carriers
- Viral Vectors
- Therapeutic Application
- Cardiovascular Diseases
- Genetic Disorders
- Duchenne Muscular Dystrophy (DMD)
- Spinal Muscular Atrophy (SMA)
- Infectious Diseases
- Bacterial Infections
- Viral Infections
- Inflammatory & Autoimmune Diseases
- Psoriasis
- Rheumatoid arthritis
- Neurological Disorders
- Alzheimer Disease
- Parkinson Disease
- Oncology
- Glioblastoma
- Hepatocellular Carcinoma
- Ophthalmic Diseases
- End User
- Academic & Research Institutes
- Contract Research Organizations
- Hospitals
- Pharmaceutical & Biotechnology Companies
- Specialty Clinics
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- ADARx Pharmaceuticals, Inc.
- Alnylam Pharmaceuticals, Inc.
- AstraZeneca PLC
- Avidity Biosciences
- BioNTech SE
- bluebird bio, Inc.
- Comanche Biopharma Corp.
- Creyon Bio, Inc.
- Eleven Therapeutics
- Ferring B.V.
- GenScript Biotech Corporation
- Ionis Pharmaceuticals, Inc.
- Moderna, Inc.
- Nogra Pharma Limited
- Novartis AG
- Novo Nordisk A/S
- OliX Pharmaceuticals, Inc.
- Pfizer Inc.
- Ractigen Therapeutics
- Regulus Therapeutics Inc.
- SANOFI WINTHROP INDUSTRIE
- Sarepta Therapeutics, Inc.
- Silence Therapeutics
- Sirnaomics Ltd.
- Stoke Therapeutics, Inc.
- Switch Therapeutics
- Tevard Biosciences
- Wave Life Sciences Ltd.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Small Nucleic Acid Therapies market report include:- ADARx Pharmaceuticals, Inc.
- Alnylam Pharmaceuticals, Inc.
- AstraZeneca PLC
- Avidity Biosciences
- BioNTech SE
- bluebird bio, Inc.
- Comanche Biopharma Corp.
- Creyon Bio, Inc.
- Eleven Therapeutics
- Ferring B.V.
- GenScript Biotech Corporation
- Ionis Pharmaceuticals, Inc.
- Moderna, Inc.
- Nogra Pharma Limited
- Novartis AG
- Novo Nordisk A/S
- OliX Pharmaceuticals, Inc.
- Pfizer Inc.
- Ractigen Therapeutics
- Regulus Therapeutics Inc.
- SANOFI WINTHROP INDUSTRIE
- Sarepta Therapeutics, Inc.
- Silence Therapeutics
- Sirnaomics Ltd.
- Stoke Therapeutics, Inc.
- Switch Therapeutics
- Tevard Biosciences
- Wave Life Sciences Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
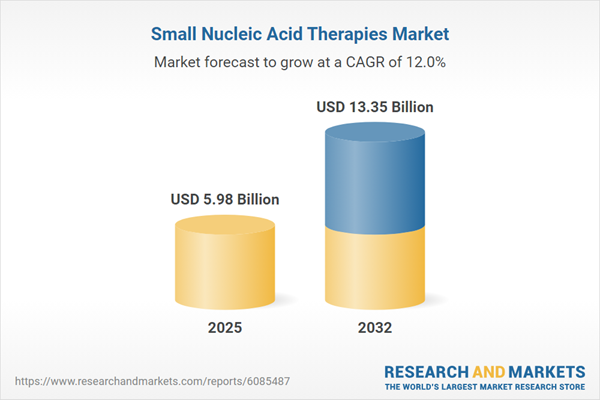
| Estimated Market Value ( USD | $ 5.98 Billion |
| Forecasted Market Value ( USD | $ 13.35 Billion |
| Compound Annual Growth Rate | 12.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 29 |