Speak directly to the analyst to clarify any post sales queries you may have.
Transarterial Chemoembolization (TACE) is increasingly central to targeted cancer therapy, supporting advancements in interventional oncology and enabling health systems to address localized tumors with higher precision and fewer side effects. As innovations accelerate and demand grows across diverse markets, senior stakeholders face new opportunities and strategic decisions in deployment, procurement, and operational optimization of TACE solutions.
Market Snapshot: Transarterial Chemoembolization Market Overview
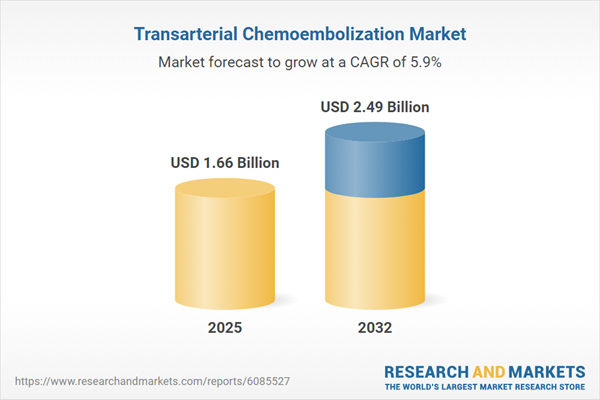
The transarterial chemoembolization market grew from USD 1.57 billion in 2024 to USD 1.66 billion in 2025. With a projected CAGR of 5.90%, it is expected to reach USD 2.49 billion by 2032. This dynamic growth underscores robust clinical adoption and ongoing innovation in TACE devices, chemotherapeutics, and interventional delivery systems. Establishing a firm position within oncology portfolios, TACE’s market trajectory reflects both rising incidence rates of solid tumors and improving multidisciplinary care frameworks.
Scope & Segmentation of the Transarterial Chemoembolization Market
This report delivers targeted, actionable analyses across product, indication, procedure, end user, regional, and competitive dimensions, empowering market leaders to align strategy with the latest developments.
- Drug Types:
- Cisplatin
- Doxorubicin - Indications:
- Breast Cancer
- Liver Cancer
- Lung Cancer - Procedure Types:
- Conventional TACE
- Drug-Eluting Beads - End Users:
- Ambulatory Surgical Centers
- Hospitals
- Specialty Clinics - Regional Coverage:
- North America (United States, Canada, Mexico)
- Latin America (Brazil, Argentina, Chile, Colombia, Peru)
- Europe (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland)
- Middle East (United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel)
- Africa (South Africa, Nigeria, Egypt, Kenya)
- Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan) - Major Industry Players:
- AdvaCare Pharma, Cadila Healthcare Limited, Dr. Reddy's Laboratories Ltd., Johnson & Johnson Services, Inc., LGM Pharma, MicroBiopharm Japan Co., Ltd., Novartis AG, Pfizer Inc., Veranova, L.P., WG Critical Care, LLC
Key Takeaways for Senior Decision-Makers
- TACE is now a mainstream option for multidisciplinary oncology care, enabled by advances in imaging, microcatheters, and embolic materials.
- Drug-eluting bead technology and improved molecular diagnostics are facilitating more customized and effective patient treatment plans.
- Market expansion is shaped by global collaboration among device manufacturers, clinicians, and academic institutions, accelerating adoption and protocol refinement.
- Regional growth varies: mature healthcare systems integrate TACE rapidly, while emerging markets scale up through investment and partnerships.
- Stakeholders who prioritize procurement resilience, clinical training, and digital health integration can gain competitive differentiation in a shifting regulatory climate.
- Strategic alliances, expanded service offerings, and robust clinical validation are crucial for sustained market leadership and differentiation.
Tariff Impact on Supply Chain and Cost Structures
Recent United States tariff revisions on medical devices and pharmaceutical inputs are compelling procurement teams, manufacturers, and healthcare administrators to adapt sourcing strategies and renegotiate contracts. These pressures drive exploration of alternate suppliers, localized production, and innovative supply chain models, ensuring uninterrupted availability of high-impact TACE materials while maintaining procedural standards and budget control.
Methodology & Data Sources
This analysis is founded on a blended approach featuring secondary research from peer-reviewed journals, clinical registries, and regulatory filings. Direct interviews with radiologists, oncologists, procurement specialists, and regulatory experts supplement data modeling and qualitative trend analysis to validate findings and contextualize insights.
Why This Report Matters
- Guides resource allocation by illuminating high-growth segments and underlying drivers in each region and product area.
- Enables benchmarking of competitive strategies, procurement frameworks, and partnership models across global operators.
- Assists clinical and commercial leaders in evaluating technology integration, patient selection advancement, and operational best practices to support superior patient outcomes.
Conclusion
This report serves as a comprehensive resource for navigating the evolving transarterial chemoembolization landscape. It supports strategic planning and informed investment for sustained success in targeted interventional oncology.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Transarterial Chemoembolization market report include:- AdvaCare Pharma
- Cadila Healthcare Limited
- Dr. Reddy’s Laboratories Ltd.
- Johnson & Johnson Services, Inc.
- LGM Pharma
- MicroBiopharm Japan Co., Ltd.
- Novartis AG
- Pfizer Inc.
- Veranova, L.P.
- WG Critical Care, LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 1.66 Billion |
| Forecasted Market Value ( USD | $ 2.49 Billion |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |