Global Cell and Gene Therapy Third-Party Logistics Industry Overview
Worldwide gene and cell treatment the growing need for customized medicine and technological breakthroughs are fueling the third-party logistics (3PL) sector's rapid expansion. These treatments frequently include biological components that are sensitive to temperature, such live cells and viral vectors, which call for particular handling, storage, and transit techniques. Logistics companies are investing in cutting-edge cold chain infrastructure, such as cryogenic shippers, ultra-low temperature freezers, and real-time tracking systems, to meet these demands. Manufacturers and logistics firms are increasingly working together to optimize distribution networks and guarantee on-time delivery. With advancements in supply chain optimization and patient-centric logistics improving the effectiveness and dependability of cell and gene therapy distribution, the sector is well-positioned for further growth despite obstacles like regulatory complexity and the requirement for specialized storage facilities.Additionally, one of the key drivers of market expansion is the growing number of clinical studies and regulatory approvals for gene and cell treatments. The demand for safer and more effective transportation is being driven by the growing number of CGTs that are progressing from research to clinical development and ultimately commercialization. In order to move temperature-sensitive treatments, pharmaceutical and biotech businesses are carrying out multi-site studies across several areas, necessitating sophisticated logistical solutions.
Logistics are further complicated by the fact that cell and gene treatments sometimes entail direct-to-patient shipments or need patients to go to specialist treatment facilities. Several of these studies concentrate on orphan and uncommon illnesses, necessitating the coordination of delivery to sites with limited access or remote locations by logistics companies. The need for logistics firms to provide flexible and scalable solutions has grown as a result of the growing number of investigational new drug (IND) applications for CGTs. In order to improve the safe and effective transportation of these extremely sensitive treatments, third-party logistics (3PL) providers are being asked to provide specialized services like sophisticated packaging options and ultra-cold storage facilities, which are becoming more and more necessary as cell and gene therapy clinical trials expand quickly.
Growth Drivers for the Cell and Gene Therapy Third-Party Logistics Market
Rising Demand for Cell and Gene Therapies
One of the main factors propelling the biotechnology and pharmaceutical industries' expansion is the growing need for cell and gene treatments. Personalized therapies based on each patient's unique genetic profile are becoming increasingly common as medical research advances. These treatments frequently include extremely delicate biological components that need specific handling, storage, and shipping, such live cells and viral vectors. Because these treatments are individualized and complicated, using specialist logistics solutions is necessary to guarantee the timely and secure delivery of goods. In order to achieve these needs, third-party logistics providers are crucial because they provide sophisticated tracking systems, temperature-controlled conditions, and regulatory compliance. The demand for advanced logistics solutions will only rise as customized medicine expands.Growing Investment in Biotech and Healthcare
The healthcare industry is expanding significantly as a result of increased biotechnology investment and the creation of novel cell and gene treatments. In order to reach a worldwide market, newly developed treatments frequently need increasingly intricate and sophisticated distribution networks. The demand for specialized logistics that can handle physiologically complex and temperature-sensitive items arises from the move toward customized medicine and advanced therapies. Therapy developers are establishing strategic alliances with third-party logistics (3PL) suppliers in order to satisfy these increasing needs. These partnerships facilitate international supply chains, guaranteeing the safe and effective cross-border delivery of treatments. The need for skilled logistics services to handle these cutting-edge treatments will only increase as biotech and healthcare investments continue to grow.Patient-Centric Logistics
The emphasis of logistics is moving toward patient-centric solutions as medicines become more customized. Cell and gene therapy patients need a customized strategy, which frequently includes door-to-door delivery to guarantee the product gets to them safely and on schedule. By providing real-time tracking systems, third-party logistics companies are adjusting and giving patients the most recent information on the progress of their therapeutic shipment. By guaranteeing transparency and lowering ambiguity, this improves the patient experience overall. To preserve the integrity of the treatments while in transit, logistics companies are also spending money on sophisticated packaging, temperature control, and accelerated delivery. Patient-centric logistics is becoming a crucial component in the effective delivery of vital medications as customized medicine gains traction.Challenges in the Cell and Gene Therapy Third-Party Logistics Market
Packaging and Handling Challenges
Since cell and gene treatments frequently need exact temperature control and safe storage to preserve their integrity, handling and packaging them can be quite difficult. In order to prevent product degradation and make the therapy hazardous or ineffective, proper packaging must protect against temperature changes, contamination, and physical damage. Because of their sensitivity, these biologics require specific materials and monitoring systems to guarantee product stability from manufacturing to delivery. Robust packaging solutions are an essential part of the therapeutic process since improper handling and packing can raise the chance of treatment failures in addition to jeopardizing the therapy's safety and effectiveness.Temperature Sensitivity
Live cells or viral vectors, which are extremely sensitive to temperature changes, are frequently used in cell and gene treatments. Strict temperature control is necessary for these treatments to maintain their biological activity and guarantee efficacy. To avoid deterioration or loss of efficacy during transportation, especially over long distances, a constant temperature must be maintained. In order to track and manage temperature conditions during the whole shipping process, this logistical problem necessitates the use of specialist packing solutions, such as temperature-controlled containers and real-time monitoring systems. Any failure to maintain proper temperature control might result in a weakened product, endangering patient safety and therapeutic results. As a result, protecting these treatments from temperature changes is essential to guaranteeing their effective administration.United States Cell and Gene Therapy Third-Party Logistics Market
The third-party logistics (3PL) business for cell and gene therapy in the United States is essential to meeting the intricate supply chain needs of these cutting-edge treatments. 3PL suppliers are required to provide highly specialized services, such as temperature-controlled shipping, real-time tracking, regulatory compliance, and secure storage solutions, since cell and gene products are delicate. By assisting producers in overcoming the difficulties associated with managing live biological materials, these logistics partners guarantee the prompt and secure transportation of goods from manufacturing sites to treatment facilities. Agile, compliant, and technologically sophisticated logistics solutions are becoming increasingly important as the need for tailored medicine rises. In order to facilitate the scalability, dependability, and effectiveness of cell and gene therapy distribution throughout the United States, third-party logistics companies are becoming more and more crucial.Germany Cell and Gene Therapy Third-Party Logistics Market
The emerging biopharmaceutical industry in Germany, which is distinguished by its sophisticated infrastructure and strict regulatory requirements, depends heavily on the country's cell and gene therapy third-party logistics (3PL) business. For the safe and legal shipment of temperature-sensitive treatments, such CAR-T cells and viral vectors, specialized logistics providers are crucial. For these treatments to remain effective, careful cold chain management is necessary, including secure storage and real-time monitoring. The market is expanding significantly due to a rise in clinical studies and the approval of gene and cell treatments. Western Germany is the region with the highest demand for these specialist logistics services, including biotech centers like Frankfurt and Düsseldorf. Leading companies in the industry that provide comprehensive, patient-focused supply chain solutions are UPS (Marken), Arvato, Cryoport, and AmerisourceBergen. The efficiency and dependability of logistics operations in this industry are being further improved by technological developments like AI-driven route optimization and creative packaging materials.China Cell and Gene Therapy Third-Party Logistics Market
The market for third-party logistics for cell and gene therapy is growing quickly in China because to the nation's growing investments in biotechnology and customized medicine. To maintain the integrity and effectiveness of delicate biological materials, the logistics of these treatments require specific services, such as temperature-controlled transit, safe storage, and real-time monitoring. In order to manage the extensive supply chains related to cell and gene treatments and navigate the complex regulatory environment, third-party logistics companies are crucial. Technological developments that improve efficiency and dependability, such AI-driven route optimization and IoT-based tracking systems, further assist the market's growth. Specialized logistics providers are becoming more and more important to the effective delivery of these cutting-edge treatments as China continues to solidify its place in the global biopharmaceutical market.Saudi Arabia Cell and Gene Therapy Third-Party Logistics Market
Saudi Arabia is committed to becoming a worldwide biotechnology powerhouse by 2040, which is driving the country's rapid evolution in the cell and gene therapy third-party logistics (3PL) business. Investments in cold chain logistics and other biopharmaceutical infrastructure are being propelled by the government's Vision 2030 effort in order to facilitate the effective and safe delivery of temperature-sensitive treatments. In order to improve domestic capabilities and lessen dependency on foreign supply chains, strategic alliances like the one between Vertex and the Ministry of Industry and Mineral Resources seek to localize gene therapy production. Logistics companies are being forced by this change to use cutting-edge technologies like blockchain, IoT, and AI in order to maintain compliance and streamline operations. Specialized 3PL services that can handle the challenges of moving live cells and viral vectors are in greater demand as the industry expands, guaranteeing prompt and safe delivery to treatment facilities throughout the Kingdom.Recent Developments in Cell and Gene Therapy Third-Party Logistics Industry
- Cardinal Health (NYSE: CAH) launched Advanced Therapy Connect, the first-to-market unified ordering platform for cell and gene treatments, in January 2025 through its Advanced Therapy Solutions subsidiary. In the past, ordering cell and gene therapies has required healthcare practitioners to register for many product platforms with various reporting formats, invoicing, and payment processes.
- In October 2024, McKesson Corporation announced the creation of InspiroGeneTM by McKesson ('InspiroGene'), a specialist firm, to aid in the commercialization of cell and gene therapies (CGTs). InspiroGene's scalable and flexible package of services, which includes third-party logistics programs and a skilled leadership team, may help manufacturers, payers, and providers effectively navigate the difficult terrain of CGT commercialization. This makes it more likely that patients will be able to get the transformative therapies they need.
Cell and Gene Therapy Third-Party Logistics Market Segment
Type
- Clinical
- Commercial
Product
- Cell Therapies
- Gene Therapies
Therapeutic Area
- Oncology
- Neurology
- Cardiovascular Diseases
- Ophthalmology
- Infectious Diseases
- Others
End Use
- Biopharmaceutical Companies
- CDMOs/CMOs
- Others
Country
North America
- United States
- Canada
Europe
- France
- Germany
- Italy
- Spain
- United Kingdom
- Belgium
- Netherlands
- Turkey
Asia-Pacific
- China
- Japan
- India
- Australia
- South Korea
- Thailand
- Malaysia
- Indonesia
- New Zealand
Latin America
- Brazil
- Mexico
- Argentina
Middle East & Africa
- South Africa
- Saudi Arabia
- United Arab Emirates
The key players have been analyzed by:
- Overview
- Key Persons
- Recent Development & Strategies
- Revenue Analysis
Key Players Analyzed:
- Cencora Corporation
- Cardinal Health
- McKesson Corporation
- EVERSANA
- Knipper Health
- Arvato SE
- DHL
- Kuehne+Nagel
Table of Contents
Companies Mentioned
- Cencora Corporation
- Cardinal Health
- McKesson Corporation
- EVERSANA
- Knipper Health
- Arvato SE
- DHL
- Kuehne+Nagel
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | May 2025 |
| Forecast Period | 2024 - 2033 |
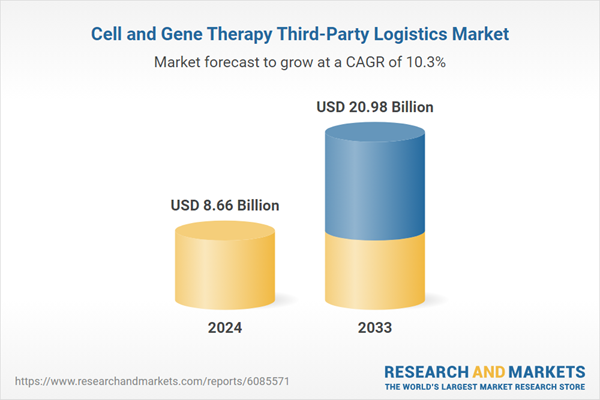
| Estimated Market Value ( USD | $ 8.66 Billion |
| Forecasted Market Value ( USD | $ 20.98 Billion |
| Compound Annual Growth Rate | 10.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 8 |