Global Prostate Cancer Biomarkers Industry Overview
The rising incidence of prostate cancer and improvements in diagnostic technology are driving growth in the global market for prostate cancer biomarkers. Prostate cancer biomarkers are essential for early detection, prognosis prediction, and therapy monitoring. These biomarkers, which aid in identifying high-risk individuals, assessing the severity of the disease, and directing treatment choices, include genetic markers, protein biomarkers, and molecular imaging agents. The need for more accurate and individualized diagnostics is being driven by the rise in prostate cancer incidence, an older population, and increased awareness of cancer detection. Furthermore, the market is expanding due to the growing trend for non-invasive diagnostic techniques.The landscape of prostate cancer biomarkers is changing due to technological developments including liquid biopsies, next-generation sequencing (NGS), and analysis powered by artificial intelligence (AI). When compared to conventional biopsy-based diagnostics, these advancements provide earlier and more accurate detection approaches. Leading companies in the field are concentrating on creating new biomarkers and raising diagnostic test accuracy. Prostate cancer biomarker adoption is also being accelerated by the increased focus on personalized medicine, which involves customizing therapies based on a patient's genetic profile. The sector is anticipated to grow as research advances and more widely available and efficient biomarker-based diagnostic technologies become available.
Prostate cancer diagnosis and treatment are being revolutionized by the growing emphasis on biomarker-driven diagnostics. Improved risk categorization and individualized treatment strategies are supported by sophisticated biomarker testing. OncoAssure Ltd. declared in January 2025 that OncoAssure Prostate, a biopsy-based test intended to improve risk assessment for prostate cancer recurrence, has been clinically validated. In order to promote more accurate treatment decisions, the study, which was published in BJUI Compass, showed that the test could differentiate between aggressive and low-risk prostate tumors. These developments highlight the expanding contribution of biomarker-based diagnostics to better clinical outcomes and the development of more efficient management plans for prostate cancer.
Growth Drivers for the Prostate Cancer Biomarkers Market
Rising Incidence of Prostate Cancer
The market for prostate cancer biomarkers is significantly influenced by the rising prevalence of prostate cancer worldwide, particularly in older populations. The necessity for early detection and individualized treatment options increases with the prevalence of prostate cancer. Early screening is essential for successful treatment and better patient outcomes since prostate cancer frequently manifests in its early stages without any discernible symptoms. By identifying high-risk patients and assessing the severity of the condition, diagnostic biomarkers assist medical practitioners customize treatment regimens. Reducing death rates and enhancing patient quality of life depend on early diagnosis using biomarkers. The need for efficient biomarkers to direct early detection and treatment is anticipated to increase as the number of prostate cancer patients keeps rising.Advancements in Diagnostic Technologies
The market for prostate cancer biomarkers is changing as a result of advancements in diagnostic technologies such liquid biopsies, molecular imaging, and next-generation sequencing (NGS). Early and more precise diagnosis is made possible by NGS's ability to identify genomic abnormalities and changes linked to prostate cancer. By examining blood samples for genetic material from tumor cells, liquid biopsy provides a non-invasive substitute for standard biopsy, making it simpler to track the course of the illness and the effectiveness of treatment. Molecular imaging tools improve the capacity to follow tumor development and metastasis by seeing the existence and spread of cancer in the body. The market for biomarkers has grown significantly as a result of these technical developments, which offer more accurate, early-stage diagnosis and real-time monitoring, lessen the need for intrusive procedures, and improve individualized treatment plans.Shift Towards Personalized Medicine
The market for prostate cancer biomarkers is expanding due in large part to the trend toward customized therapy. The use of personalized therapies, which are made to fit each patient's particular genetic profile, is growing in the treatment of prostate cancer. In order to create individualized treatment regimens that increase the likelihood of success, biomarkers are essential for determining which patients will react to certain medications. Biomarkers assist in dividing prostate cancer into discrete subtypes by identifying genetic mutations, protein expressions, or other molecular markers, guaranteeing that patients receive the most efficient and focused treatments. This method maximizes overall patient results, avoids needless treatments, and minimizes adverse effects. The need for biomarkers to inform treatment choices is anticipated to grow as customized medicine gets traction.Challenges in the Prostate Cancer Biomarkers Market
Regulatory and Approval Barriers
One of the biggest obstacles facing the business is the regulatory approval procedure for novel biomarkers of prostate cancer. Before being approved by regulatory agencies such as the U.S. Food and Drug Administration (FDA), biomarkers must pass stringent testing in clinical studies to demonstrate their safety, efficacy, and therapeutic relevance. The promise of novel biomarkers to transform the detection and treatment of prostate cancer may be hampered by this drawn-out and difficult approval procedure. Additionally, different locations may have different regulatory criteria, which makes it more difficult for biomarker developers to get clearance globally. Patients frequently experience delays in accessing potentially life-saving diagnostic tools and medications due to the lengthy regulatory processes and high costs associated with biomarker approval.High Development Costs
A significant amount of money must be spent on research, clinical trials, and technological development in order to create prostate cancer biomarkers. Finding, confirming, and commercializing biomarkers is an expensive procedure that calls for specialized knowledge, sophisticated lab equipment, and substantial funding for lengthy studies. The expenses incurred in securing regulatory permission impose an additional financial strain. Since smaller businesses frequently find it difficult to obtain the required capital, this high development cost may restrict the number of businesses that may enter the prostate cancer biomarkers industry. The development of prostate cancer diagnostics may be slowed as a result of the limited number of novel biomarkers that make it to market. Exorbitant expenses also affect price, which may restrict access for patients and healthcare providers.United States Prostate Cancer Biomarkers Market
The market for prostate cancer biomarkers in the US is expanding quickly due to factors such the rising incidence of prostate cancer, improvements in diagnostic tools, and a move toward customized treatment. The need for precise, non-invasive diagnostic methods is increasing due to the rise in prostate cancer diagnoses, which is driving the market for biomarkers. Technologies like liquid biopsies and next-generation sequencing (NGS) are enhancing therapy monitoring and early diagnosis. Furthermore, the usefulness of biomarkers in directing therapeutic choices is being reinforced by the movement toward personalized medicine, which customizes care according to a patient's genetic profile. When taken as a whole, these elements are speeding up the use of biomarkers, which is helping the industry expand and provide more accurate, focused diagnostic tools.Germany Prostate Cancer Biomarkers Market
The rising prevalence of prostate cancer and improvements in diagnostic technology are driving the growth of the prostate cancer biomarkers market in Germany. Effective biomarkers are increasingly needed as the need for individualized therapy and early diagnosis grows. With its emphasis on sophisticated screening initiatives, Germany's healthcare system encourages the use of cutting-edge diagnostic technologies including liquid biopsies and next-generation sequencing (NGS). The market is growing as a result of these technologies, which improve therapy monitoring and early detection. The use of biomarkers is also being accelerated by the move toward personalized medicine, which bases therapies on each patient's unique genetic profile. The market is expanding due to the healthcare infrastructure and rising knowledge of the advantages of biomarker-based diagnostics.India Prostate Cancer Biomarkers Market
The rising incidence of prostate cancer and improvements in diagnostic technology are driving the growth of the prostate cancer biomarkers market in India. Early detection and individualized treatment options are in greater demand due to the aging population and increased awareness of prostate health. Technologies like liquid biopsy and next-generation sequencing (NGS) are improving diagnostic precision and making it possible to track treatment outcomes non-invasively. Furthermore, the use of biomarkers in clinical practice is growing faster due to the trend toward personalized medicine, which customizes therapies based on each patient's unique genetic profile. The market's expansion is further supported by the Indian government's efforts to upgrade healthcare facilities and provide access to cutting-edge diagnostic equipment.Saudi Arabia Prostate Cancer Biomarkers Market
The market for prostate cancer biomarkers in Saudi Arabia is expanding gradually due to rising need for precise diagnosis and improved prostate cancer treatment options. Prostate cancer is becoming more common, especially in the elderly population, hence early detection methods are more important to enhance patient outcomes. The precision and non-invasive monitoring of the illness are being improved by developments in diagnostic tools, including as liquid biopsy and next-generation sequencing (NGS). Additionally, biomarkers are becoming increasingly important in customizing therapies for each patient as personalized medicine gains traction. It is anticipated that Saudi Arabia's initiatives to provide access to cutting-edge diagnostic equipment and improve its healthcare infrastructure would further propel market expansion by providing more effective and efficient prostate cancer management options.Recent Developments in Prostate Cancer Biomarkers Industry
- The AI-driven prostate cancer technology platform PATHOMIQ_PRAD was licensed exclusively in the United States by Myriad Genetics and PATHOMIQ in February 2025. Through this collaboration, Myriad's oncology offering will incorporate AI-enabled diagnostics, facilitating better treatment choices both prior to and during prostate cancer treatment. In line with changing demands in the market for prostate cancer biomarkers, the partnership seeks to improve diagnostic accuracy in prostate cancer treatment by utilizing cutting-edge artificial intelligence.
- DiaCarta and OncoAssure Ltd. signed a strategic partnership in February 2024 to market a test for prostate cancer prognosis. This six-gene expression test calculates the likelihood of a biochemical recurrence within five years after surgery and assesses the risk of aggressive illness after diagnosis. Through biomarker-based risk stratification, the collaboration supports the test's validation and market growth by leveraging DiaCarta's clinical diagnostic capabilities, which helps to provide more individualized prostate cancer treatment.
Prostate Cancer Biomarkers Market Segment
Type
- Genetic Biomarker
- Cell-based Biomarkers
- Metabolomic Biomarkers
Application
- Screening And Early Detection
- Diagnostic And Risk Stratification
- Prognosis And Treatment Monitoring
- Companion Diagnostics
End Use
- Hospitals & Diagnostic Laboratories
- Academic & Research Institutes
- Biopharmaceutical Companies
Country
North America
- United States
- Canada
Europe
- France
- Germany
- Italy
- Spain
- United Kingdom
- Belgium
- Netherlands
- Turkey
Asia-Pacific
- China
- Japan
- India
- Australia
- South Korea
- Thailand
- Malaysia
- Indonesia
- New Zealand
Latin America
- Brazil
- Mexico
- Argentina
Middle East & Africa
- South Africa
- Saudi Arabia
- United Arab Emirates
The key players have been analyzed by:
- Overview
- Key Persons
- Recent Development & Strategies
- Revenue Analysis
Key Players Analyzed:
- Exact Sciences Corp
- Myriad Genetics Inc
- BIO-TECHNE Corp
- OPKO HEALTH, INC.
- MDxHealth SA
- Veracyte Inc
- Roche Diagnostic Ltd.
- Pfizer Inc.
Table of Contents
Companies Mentioned
- Exact Sciences Corp
- Myriad Genetics Inc
- BIO-TECHNE Corp
- OPKO HEALTH, INC.
- MDxHealth SA
- Veracyte Inc
- Roche Diagnostic Ltd.
- Pfizer Inc.
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | May 2025 |
| Forecast Period | 2024 - 2033 |
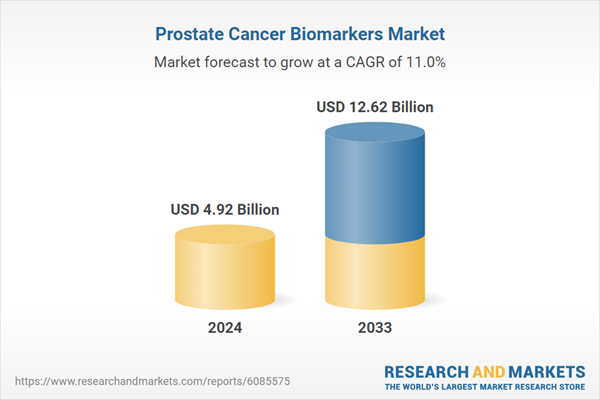
| Estimated Market Value ( USD | $ 4.92 Billion |
| Forecasted Market Value ( USD | $ 12.62 Billion |
| Compound Annual Growth Rate | 11.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 8 |