Netherlands Diabetes Devices Industry Overview
As the population ages and the frequency of diabetes rises, the Netherlands' diabetic device market is expanding steadily. In the Netherlands, more than 1.2 million individuals have diabetes, mostly type 2, as of 2025. This number is rising as a result of sedentary lifestyles, obesity, and genetic susceptibility. Innovative diabetes care solutions, especially blood glucose monitoring systems and insulin administration devices, are in high demand due to this expanding patient pool. Widespread adoption of these technologies is further supported by the nation's well-established healthcare infrastructure and advantageous reimbursement regulations.In the Netherlands, type 2 diabetes is the most common ailment, accounting for 90% of cases. In the Netherlands, where diabetes is on the rise, 58,000 new cases are recorded each year. The prevalence of diabetes was higher among those aged 70-79, with rates ranging from 3.3% to 17.9%. In the Netherlands, diabetes is one of the main causes of disease and death. In 2019, diabetes was a contributing factor in over 12,000 deaths nationwide.
The Netherlands' approach to diabetes care has changed dramatically as a result of technological breakthroughs. Smart insulin pens, integrated insulin pumps, flash glucose monitors, and continuous glucose monitoring (CGM) devices are becoming more and more popular due to their capacity to provide more precise, real-time glucose tracking and individualized therapy modifications. Leading medical device manufacturers with cutting-edge products that improve patient compliance and quality of life, like Medtronic, Abbott, and Dexcom, are present in the Dutch market. In keeping with the nation's larger commitment to digital healthcare innovation, digital health integration - including mobile applications and remote monitoring features - is also quickening.
Public awareness initiatives and government participation are also important factors influencing the diabetic devices market in the Netherlands. Through national initiatives and educational programs, the Dutch government encourages early screening, prevention, and illness management. Furthermore, partnerships between private businesses and public health organizations seek to lower the cost and increase the accessibility of cutting-edge diabetic equipment. This setting has produced a market that is innovative and competitive, which promotes ongoing R&D investment. It is anticipated that the Dutch market will continue to grow in the future, particularly as wearable and AI-enabled diabetes solutions become more popular and provide individualized, data-driven treatment plans.
Growth Drivers for the Netherlands Diabetes Devices Market
Government Initiatives and Health Awareness
The adoption of diabetic devices is directly impacted by the Dutch government's crucial role in raising diabetes management and health awareness. The government informs the people about diabetes prevention, healthy lifestyles, and early detection through nationwide public health initiatives. Regular screening programs and diabetes education campaigns are two examples of initiatives that assist people realize how important it is to monitor and manage the condition. In order to ensure greater access to cutting-edge diabetes treatment tools like insulin pumps and continuous glucose monitors (CGMs), the government also funds and supports reimbursement for healthcare technology. These programs foster innovation in healthcare by making it possible for patients and healthcare professionals to use and profit from state-of-the-art diabetic technology.Aging Population
One of the main causes of the growing prevalence of type 2 diabetes in the Netherlands is the aging population. Effective blood sugar regulation becomes more challenging as people age because their bodies' sensitivity to insulin tends to decline. Diabetes is more common in elderly persons as a result of these and other factors including poor eating habits and sedentary lives. The need for diabetes management solutions is exacerbated by the fact that type 2 diabetes is more prevalent in those over 60. The increasing number of older adults with diabetes emphasizes the need for easily accessible and efficient diabetes treatment, such as insulin pumps, glucose monitors, and educational initiatives to assist control the condition and enhance the lives of those affected.Advancements in Technology
In the Netherlands, technological developments have completely changed the way diabetes is managed, especially with regard to devices like insulin pumps, continuous glucose monitoring (CGM) systems, and remote monitoring tools. Accurate, real-time blood glucose data is provided by CGM devices, enabling patients to promptly modify their therapy and avoid harmful swings. Continuous insulin administration is made possible by insulin pumps, which give blood sugar management more flexibility and accuracy. Healthcare professionals may track patient data remotely with the use of remote monitoring systems, guaranteeing prompt interventions and individualized treatment. By providing more precise, real-time data, these technologies not only improve patient outcomes but also help people with diabetes live better lives by lessening the need for frequent manual monitoring and insulin shots.Challenges in the Netherlands Diabetes Devices Market
Healthcare System Integration Challenges
There are organizational and logistical difficulties in integrating digital health technologies into the current healthcare system. The implementation of diabetes management applications varies throughout healthcare professionals due to the absence of established recommendations. Furthermore, the lack of a cohesive strategy may lead to fragmented treatment, as patients utilize several applications that might not work with one another or with the systems of medical experts, making it more difficult to control diseases effectively.For diabetes management devices to be available, safe, and smoothly incorporated into the Dutch healthcare system, regulatory agencies, healthcare providers, and technology innovators must work together to address these issues.
Adoption Barriers Among Patients
The broad use of mobile health applications for diabetes treatment is hampered by patient adoption obstacles, especially among older persons in the Netherlands. Many people struggle with inadequate digital literacy, which can make it difficult for them to use new technology. Hesitancy is also influenced by worries about data privacy and the security of private health information, particularly in light of the increasing use of digital health solutions. Additionally, some patients are nervous while utilizing new gadgets because they worry about possible problems or technological difficulties. The complexity of modern technologies and the perceived hazards deter complete acceptance, despite the fact that many people are receptive to advancements. Better education, user-friendly designs, and unambiguous information regarding the security and advantages of these technologies are necessary to overcome these obstacles and increase acceptability.Netherlands Diabetes Devices Market Type
Types
- Self-Monitoring Devices
- Test Strips
- Lancets
- Blood Glucose Meters
- Continuous Glucose-Monitoring Devices
- Sensors
- Transmitter
- Receiver
- Insulin Pumps
- Patch Pumps
- Tethered Pumps
- Consumables
- Insulin Pens
- Disposable Insulin Pen
- Reusable Insulin Pen
End User
- Hospitals
- Diagnostics Centers
- Homecare
The key players have been analyzed by:
- Key Persons
- Recent Developments & Strategies
- Product Portfolio & Product Launch in Last 1 Year
- Revenue
Key Players Analyzed:
- Dexcom Inc
- Medtronic
- Roche
- Abbott Laboratories
- Eli Lilly
- Terumo Corporation
- BD
Table of Contents
1. Introduction2. Research & Methodology
2.1 Data Source
2.1.1 Primary Source
2.1.2 Secondary Source
2.2 Research Approach
2.2.1 Top-down Approach
2.2.2 Bottom-up Approach
2.3 Forecast Projection Methodology
3. Executive Summary
4. Market Dynamics
4.1 Growth Drivers
4.2 Challenges
5. Netherlands Diabetes Devices Market
5.1 Historical Market Trends
5.2 Market Forecast
6. Netherlands Diabetes Population
6.1 Type 1 Diabetes
6.2 Type 2 Diabetes
7. Market Share Analysis
7.1 By Types
7.2 By End User
8. Types
8.1 Self-Monitoring Devices
8.1.1 Test Strips
8.1.2 Lancets
8.1.3 Blood Glucose Meters
8.2 Continuous Glucose-Monitoring Devices
8.2.1 Sensors
8.2.2 Transmitter
8.2.3 Receiver
8.3 Insulin Pumps
8.3.1 Patch Pumps
8.3.2 Tethered Pumps
8.3.3 Consumables
8.4 Insulin Pens
8.4.1 Disposable Insulin Pen
8.4.2 Reusable Insulin Pen
9. End User - Volume
9.1 Hospitals
9.2 Diagnostics Centers
9.3 Homecare
10. Porters Five Forces
10.1 Bargaining Power of Buyer
10.2 Bargaining Power of Supplier
10.3 Threat of New Entrants
10.4 Rivalry among Existing Competitors
10.5 Threat of Substitute Products
11. SWOT Analysis
11.1 Strengths
11.2 Weaknesses
11.3 Opportunities
11.4 Threats
12. Reimbursement Policies
12.1 CGM Devices in Netherlands
12.2 Blood Glucose Devices in Netherlands
12.3 Insulin Pump Products in Netherlands
12.4 Insulin Pen in Netherlands
13. Key Players Analysis
13.1 Dexcom Inc
13.1.1 Overviews
13.1.2 Key Person
13.1.3 Recent Developments & Strategies
13.1.4 Product Portfolio & Product Launch in Last 1 Year
13.1.5 Revenue
13.2 Medtronic
13.2.1 Overviews
13.2.2 Key Person
13.2.3 Recent Developments & Strategies
13.2.4 Product Portfolio & Product Launch in Last 1 Year
13.2.5 Revenue
13.3 Roche
13.3.1 Overviews
13.3.2 Key Person
13.3.3 Recent Developments & Strategies
13.3.4 Product Portfolio & Product Launch in Last 1 Year
13.3.5 Revenue
13.4 Abbott Laboratories
13.4.1 Overviews
13.4.2 Key Person
13.4.3 Recent Developments & Strategies
13.4.4 Product Portfolio & Product Launch in Last 1 Year
13.4.5 Revenue
13.5 Eli Lilly
13.5.1 Overviews
13.5.2 Key Person
13.5.3 Recent Developments & Strategies
13.5.4 Product Portfolio & Product Launch in Last 1 Year
13.5.5 Revenue
13.6 Terumo Corporation
13.6.1 Overviews
13.6.2 Key Person
13.6.3 Recent Developments & Strategies
13.6.4 Product Portfolio & Product Launch in Last 1 Year
13.6.5 Revenue
13.7 BD
13.7.1 Overviews
13.7.2 Key Person
13.7.3 Recent Developments & Strategies
13.7.4 Product Portfolio & Product Launch in Last 1 Year
13.7.5 Revenue
Companies Mentioned
- Dexcom Inc
- Medtronic
- Roche
- Abbott Laboratories
- Eli Lilly
- Terumo Corporation
- BD
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | May 2025 |
| Forecast Period | 2024 - 2033 |
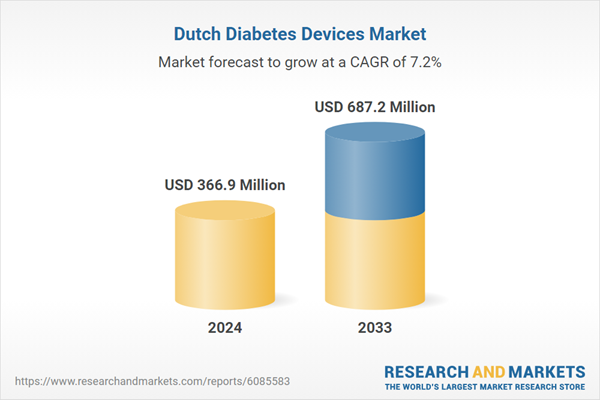
| Estimated Market Value ( USD | $ 366.9 Million |
| Forecasted Market Value ( USD | $ 687.2 Million |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Netherlands |
| No. of Companies Mentioned | 7 |