North America Balloon Catheter Industry Overview
The market for balloon catheters in North America is expanding significantly, mostly due to improvements in medical technology and the growing incidence of cardiovascular disorders. In operations like angioplasty, balloon catheters are frequently used to treat blocked or constricted arteries, especially in peripheral and coronary applications. The use of these devices is growing as a result of advancements in catheter design and the rise in cardiovascular treatments. The market is also expanding as a result of the increased emphasis on minimally invasive procedures, since balloon catheters allow doctors to treat patients more precisely, effectively, and with fewer side effects.Technological advancements like drug-coated balloon catheters, which offer tailored therapy and enhance patient outcomes, are important market drivers. Growth is also being aided by the emergence of ambulatory surgery centers (ASCs), which give patients and healthcare professionals with more affordable options. But obstacles including the high price of balloon catheters, legal restrictions, and the requirement for skilled medical personnel could impede market expansion. Notwithstanding these obstacles, it is anticipated that the North American balloon catheter market will continue to grow due to the ongoing advancement of sophisticated catheter technologies and advantageous healthcare regulations.
In the United States, heart disease continues to be the top cause of mortality for both men and women of all racial and ethnic backgrounds. According to a report published in October 2024, cardiovascular disease claims the life of a person in the US every 33 seconds. This figure is quite high and illustrates the significant impact that heart disease has on public health, which will keep driving up demand for effective cardiovascular treatments like angioplasty operations.
According to a study published in the Journal of the American College of Cardiology in February 2023, patients with elevated 10- and 30-year risk predictions have a significantly higher incidence rate of ASCVD; in at-risk individuals, this rate can reach 2.60 per 1,000 person-years. The rising prevalence of cardiovascular disease, especially in high-risk individuals, is anticipated to increase demand for angioplasty procedures and propel the market for angioplasty balloons in the United States. The region's expanding angioplasty balloon market is bolstered by the ongoing need for interventional therapies to treat atherosclerosis.
Growth Drivers for the North America Balloon Catheter Market
Rising Prevalence of Cardiovascular Diseases
One of the main factors propelling the balloon catheter market in North America is the increasing incidence of cardiovascular diseases (CVDs). Due to factors including aging populations, poor lifestyle choices, and rising rates of diabetes and hypertension, conditions like peripheral artery disease (PAD) and coronary artery disease (CAD) are becoming increasingly prevalent. Angioplasty operations, which enlarge blocked or restricted arteries to improve blood flow, require balloon catheters as vital tools. The need for efficient, minimally invasive procedures like angioplasty is growing as the number of people with CVDs keeps rising. The region's market is expanding as a result of the increasing demand for intervention, which encourages hospitals and ambulatory surgery centers to use balloon catheters.Improved Healthcare Infrastructure
The sophisticated healthcare system in North America is a major factor propelling the balloon catheter market's expansion. Modern hospitals, clinics, and research facilities with the newest technology for identifying and treating cardiovascular diseases can be found in the area. Furthermore, the growing number of qualified medical specialists, such as cardiologists and interventional specialists, guarantees the effectiveness and high success rates of balloon catheter-based operations like angioplasty. The need for balloon catheters is further increased by the broad availability of these facilities and specialists, which improves patient access to high-quality care. North America's robust infrastructure will continue to play a significant role in the market's growth as healthcare systems continue to change.Increase in Ambulatory Surgical Centers (ASCs)
The market for balloon catheters in North America is expanding due to the rise in ambulatory surgical centers (ASCs). For patients in need of minimally invasive procedures like angioplasty, ASCs provide a convenient and economical alternative to typical hospital settings. With their emphasis on outpatient care, these facilities offer quicker recovery periods and less hospital stays, which appeals to patients and medical professionals alike. The need for balloon catheters is being fueled by the increasing inclination for outpatient operations as well as the growth in ASCs. In order to execute cardiovascular procedures, ASCs are increasingly using cutting-edge medical technology, such as balloon catheters, which helps to grow the market for these devices.Challenges in the North America Balloon Catheter Market
High Cost of Devices
A major obstacle in the North American market is the expensive cost of balloon catheters, particularly those with sophisticated features like drug-coated alternatives. Although these specialty catheters have improved therapeutic benefits, like lowering the incidence of restenosis, their higher cost may prevent some people from using them, especially in healthcare settings where money is tight. The expense might be a significant deterrent for patients with low insurance coverage, outpatient clinics, and smaller hospitals. Because of this financial barrier, patients and healthcare professionals may choose less costly options, delaying the adoption of novel catheter technology. Therefore, the expensive cost of balloon catheters may limit their broad use and inhibit overall market growth, even in spite of the clinical benefits.Complex Procedures and Skill Requirements
Widespread adoption is hampered by the complexity of operations involving balloon catheters, which call for qualified medical personnel. High degrees of accuracy and skill are required for certain operations, including angioplasty, in order to guarantee success and reduce patient risks. Because of this, doctors and medical personnel must undergo specialized training, which may reduce the number of trained experts available in smaller or less well-equipped healthcare facilities. The use of balloon catheters may be limited by the requirement for sophisticated skill sets and specific equipment, particularly in impoverished or rural locations. The adoption of balloon catheter-based treatments may be hampered by this lack of infrastructure and general expertise, which could limit patient access to potentially life-saving operations and delay the market's overall growth.United States Balloon Catheter Market
The market for balloon catheters in the US is expanding significantly because to the increased prevalence of cardiovascular conditions such peripheral artery disease and coronary artery disease. In operations like angioplasty, which open clogged or constricted arteries, balloon catheters are essential instruments. The demand for balloon catheters is further increased by the growing preference for less invasive procedures, which provide fewer complications and quicker recovery times. Treatment results are also being improved by developments in catheter technology, including as drug-coated and balloon-expandable models. High levels of medical competence, robust reimbursement policies, and a well-developed healthcare infrastructure all support the U.S. market. The market is anticipated to keep growing in the upcoming years despite obstacles including high device costs and regulatory barriers.Canada Balloon Catheter Market
The rising incidence of cardiovascular disorders and improvements in medical technology are fueling the balloon catheter market's steady expansion in Canada. In operations like angioplasty, balloon catheters are vital instruments that efficiently treat clogged or constricted arteries. A strong healthcare infrastructure and an increasing number of qualified experts who guarantee the efficient use of these technologies assist the market. Further encouraging the use of balloon catheter treatments is the growth of ambulatory surgery centers (ASCs), which provide affordable substitutes for conventional hospital settings. The Canadian balloon catheter market is expected to continue expanding due to ongoing technical advancements and rising demand for minimally invasive treatments, despite obstacles such high device costs and complicated regulations.Mexico Balloon Catheter Market
The market for balloon catheters in Mexico is expanding due to rising rates of cardiovascular disease and improvements in medical technology. In treatments for clogged or constricted arteries, such as angioplasty, balloon catheters are crucial. A strong healthcare infrastructure and an increasing number of qualified experts who guarantee the efficient use of these technologies assist the market. Further encouraging the use of balloon catheter treatments is the growth of ambulatory surgery centers (ASCs), which provide affordable substitutes for conventional hospital settings. The Mexican balloon catheter market is expected to grow further due to ongoing technical advancements and rising demand for minimally invasive treatments, even in the face of obstacles including high device costs and complicated regulations.North America Balloon Catheter Market Segmentation
Product Type-Market breakup in 6 viewpoints:
- Normal Balloon Catheter
- Drug Eluting Balloon Catheter
- Cutting Balloon Catheter
- Scoring Balloon Catheter
- Stent Graft Balloon Catheter
- Others
Indication Market breakup in 2 viewpoints:
- Coronary Artery Disease
- Peripheral Vascular Disease
Raw Material Market breakup in 3 viewpoints:
- Polyurethane
- Nylon
- Others
End Users Market breakup in 4 viewpoints:
- Hospitals
- Clinics
- Ambulatory Surgical Centers
- Diagnostic Centers
Country -Market breakup in 4 viewpoints:
- United States
- Canada
- Mexico
- Rest of North America
The key players have been analyzed by:
- Overviews
- Key Persons
- Recent Developments
- Revenue
Key Players Analyzed:
- Abbott Laboratories
- Medtronic Plc.
- B.Braun Melsungen AG
- Terumo Corporation
- Cordis Corporation
- Becton Dickinson And Company
- Cardinal Health
- Stryker Corporation
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Medtronic Plc.
- B.Braun Melsungen AG
- Terumo Corporation
- Cordis Corporation
- Becton Dickinson And Company
- Cardinal Health
- Stryker Corporation
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | May 2025 |
| Forecast Period | 2024 - 2033 |
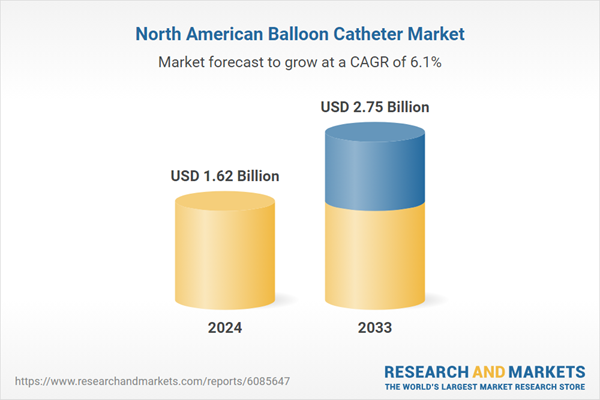
| Estimated Market Value ( USD | $ 1.62 Billion |
| Forecasted Market Value ( USD | $ 2.75 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | North America |
| No. of Companies Mentioned | 8 |