Global Implantable Loop Recorders Market - Key Trends & Drivers Summarized
What Is Driving the Clinical Relevance of Implantable Loop Recorders in Modern Cardiology?
Implantable loop recorders (ILRs), also known as insertable cardiac monitors (ICMs), have revolutionized long-term cardiac rhythm monitoring by enabling continuous ECG surveillance for up to three years. These subcutaneous devices are particularly valuable in diagnosing unexplained syncope, cryptogenic stroke, and intermittent arrhythmias, such as paroxysmal atrial fibrillation. Unlike traditional Holter monitors or external event recorders, ILRs eliminate the limitations of short-term data capture and patient non-compliance with wearable electrodes. This ability to provide uninterrupted cardiac monitoring, even in asymptomatic patients, significantly enhances diagnostic yield, especially in cases where arrhythmic episodes are infrequent or unpredictable.Clinical guidelines from the American Heart Association (AHA) and European Society of Cardiology (ESC) increasingly recommend ILRs as first-line tools in patients with unexplained syncope or transient loss of consciousness, further legitimizing their place in cardiac diagnostic pathways. Beyond syncope, there is growing utilization of ILRs in post-stroke evaluation for cryptogenic causes, where undetected atrial fibrillation may be an underlying factor. These devices are also proving critical in long-term monitoring for patients with inherited arrhythmia syndromes or post-ablation monitoring for recurrence of atrial fibrillation, expanding their utility in both general cardiology and electrophysiology subspecialties.
How Are Technology Advancements Reshaping the Implantable Loop Recorder Landscape?
The past few years have witnessed rapid innovation in ILR design and data handling capabilities. New-generation devices are increasingly miniaturized, facilitating minimally invasive insertion procedures that can be performed in outpatient settings. For instance, some devices now measure less than 1 cubic centimeter in volume and can be implanted in under five minutes. These miniaturized form factors not only improve patient comfort but also reduce procedural risk and cost burden on healthcare systems. Battery longevity has also improved significantly, with most devices now lasting between three and four years without replacement.On the digital front, integration with remote telemetry systems and smartphone-compatible patient apps has dramatically improved real-time data access and clinical workflow. Physicians can now receive instant alerts for critical events such as asystole, bradycardia, or atrial fibrillation episodes, enabling earlier interventions. In parallel, artificial intelligence and machine learning algorithms are being embedded into cloud-based platforms to reduce false positives and automate pattern recognition across large datasets. These capabilities are especially beneficial in remote patient monitoring programs, telecardiology services, and in managing chronic disease populations outside traditional clinical settings.
Where Are the Adoption Hotspots and Which Clinical Applications Are Expanding?
North America and Europe currently lead global ILR adoption, driven by well-established reimbursement policies, strong clinical infrastructure, and early integration of remote monitoring technologies. However, emerging markets in Asia-Pacific and Latin America are witnessing a rise in implant volumes due to improved access to cardiac diagnostics, rising awareness of atrial fibrillation-related stroke, and increased investment in public cardiac health initiatives. Governments and insurers are also starting to recognize the cost-effectiveness of ILRs in reducing recurrent hospitalizations and stroke-related disability.In terms of clinical use, while syncope diagnosis remains the leading indication, ILRs are seeing rapid uptake in secondary stroke prevention programs. Many stroke centers are incorporating ILRs into their diagnostic workup to detect occult paroxysmal atrial fibrillation - a common but underdiagnosed cause of embolic strokes. Furthermore, their role is expanding in electrophysiology for post-ablation surveillance and in structural heart disease management, particularly among patients with implanted cardiac devices like pacemakers or CRTs, where ILRs provide adjunctive diagnostic insights. Pediatric cardiology is another frontier, where ILRs are gaining interest for evaluating inherited rhythm disorders in younger populations, albeit with careful selection criteria due to anatomical and procedural considerations.
The Growth in the Implantable Loop Recorders Market Is Driven by Several Factors…
First, continuous innovation in device miniaturization and minimally invasive insertion techniques has significantly lowered the threshold for adoption across both high- and middle-income countries. This has opened new avenues for use in ambulatory and community-based cardiac care models. Second, the integration of remote monitoring technologies and cloud-based analytics platforms has turned ILRs into connected health tools, aligning well with the global shift toward digital health, decentralized care, and telemonitoring. These advancements allow cardiologists to efficiently manage larger patient cohorts while maintaining diagnostic precision.Third, the expanding clinical use cases - particularly in cryptogenic stroke diagnostics, atrial fibrillation surveillance, post-ablation monitoring, and inherited rhythm disorder detection - are collectively enlarging the addressable patient pool. Additionally, supportive reimbursement policies in key markets such as the U.S., Germany, and Japan have been critical in driving both physician adoption and patient access. Fourth, rising global cardiovascular disease prevalence, especially atrial fibrillation and stroke, is fueling the need for early and reliable rhythm diagnostics, creating sustained demand for ILRs. Finally, market growth is also bolstered by the increasing availability of these devices in emerging regions, thanks to growing awareness, improving cardiology infrastructure, and targeted distribution strategies by leading device manufacturers.
Report Scope
The report analyzes the Implantable Loop Recorders market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Manual Product, Automatic Product); Indication (Cardiovascular Syncope Indication, Stroke Indication, Heart Failure Indication, Cardiac Arrhythmia Indication, Atrial Fibrillation Indication, Bundle Branch Block Indication, Other Indications); End-User (Hospitals End-User, Cardiac Centers & Clinics End-User, Ambulatory Surgery Centers End-User).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Manual Product segment, which is expected to reach US$1.7 Billion by 2030 with a CAGR of a 10.3%. The Automatic Product segment is also set to grow at 6.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $417.3 Million in 2024, and China, forecasted to grow at an impressive 14.2% CAGR to reach $572 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Implantable Loop Recorders Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Implantable Loop Recorders Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Implantable Loop Recorders Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Air Power Inc., Atlas Copco, Chicago Pneumatic Tool Company, DEWALT, FERM Power Tools and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Implantable Loop Recorders market report include:
- Abbott Laboratories
- Angel Medical Systems, Inc.
- Biotronik SE & Co. KG
- Boston Scientific Corporation
- Cardiac Insight, Inc.
- Cardiovascular Systems, Inc.
- ConMed Corporation
- Cook Medical
- GE Healthcare
- HeartWare International, Inc.
- Hill-Rom Holdings, Inc.
- iRhythm Technologies, Inc.
- Koninklijke Philips N.V.
- LifeWatch Technologies
- Medtronic plc
- MicroPort Scientific Corporation
- Nihon Kohden Corporation
- Philips Healthcare
- Preventice Solutions
- ProgenyHealth
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Angel Medical Systems, Inc.
- Biotronik SE & Co. KG
- Boston Scientific Corporation
- Cardiac Insight, Inc.
- Cardiovascular Systems, Inc.
- ConMed Corporation
- Cook Medical
- GE Healthcare
- HeartWare International, Inc.
- Hill-Rom Holdings, Inc.
- iRhythm Technologies, Inc.
- Koninklijke Philips N.V.
- LifeWatch Technologies
- Medtronic plc
- MicroPort Scientific Corporation
- Nihon Kohden Corporation
- Philips Healthcare
- Preventice Solutions
- ProgenyHealth
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 376 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
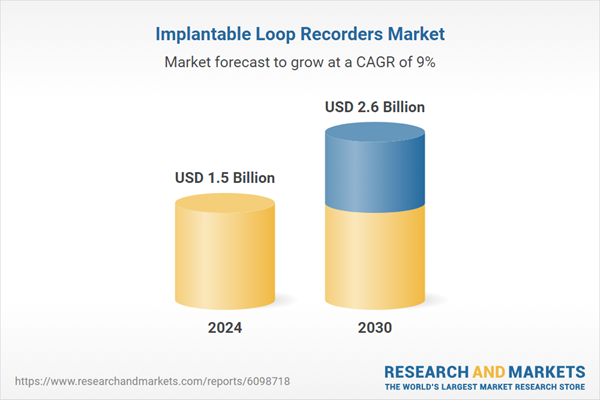
| Estimated Market Value ( USD | $ 1.5 Billion |
| Forecasted Market Value ( USD | $ 2.6 Billion |
| Compound Annual Growth Rate | 9.0% |
| Regions Covered | Global |