Global Inactivated Polio and Rabies Vaccines Market - Key Trends & Drivers Summarized
Why Are Inactivated Vaccines Regaining Importance in Global Immunization Programs?
Inactivated polio and rabies vaccines continue to play an indispensable role in global immunization strategies, particularly in the context of disease eradication and post-exposure prophylaxis. Inactivated polio vaccines (IPV), which contain a chemically inactivated version of all three poliovirus serotypes, are now the preferred option in most national immunization programs due to the risks associated with oral polio vaccine (OPV), including vaccine-derived poliovirus outbreaks. IPV eliminates this risk while maintaining high immunogenicity, making it essential in the final phase of global polio eradication efforts led by the WHO and Gavi.Similarly, inactivated rabies vaccines remain the gold standard for both pre- and post-exposure prophylaxis. These vaccines are derived from cell culture or embryonated egg platforms and are entirely non-infectious, ensuring safety even in vulnerable populations such as children and pregnant women. Their adoption is critical in endemic countries across Asia and Africa, where rabies remains a significant public health concern due to high rates of canine transmission. Both vaccine categories benefit from broad international guidelines and inclusion in WHO Essential Medicines lists, anchoring their place in public health delivery frameworks.
How Are Manufacturing Innovations and Technology Platforms Shaping Vaccine Availability?
Recent advancements in vaccine production technologies have enhanced the scalability, purity, and safety of inactivated vaccines. For IPV, modern cell culture-based manufacturing using Vero cells has replaced traditional monkey kidney cell production, yielding more consistent output and reducing contamination risks. These innovations allow large-scale production suitable for both pediatric and adult immunization programs. In response to global demand, several manufacturers have also developed combination vaccines that integrate IPV with DTaP, HepB, or Hib antigens, reducing the number of injections and improving compliance in pediatric immunization schedules.For inactivated rabies vaccines, improved purification techniques and adjuvant formulations have resulted in higher efficacy and reduced reactogenicity. Intradermal (ID) administration protocols have gained ground, especially in resource-limited settings, as they require smaller vaccine volumes without compromising immunogenicity. Cold chain optimization has also emerged as a crucial trend, with thermostable formulations under development that can tolerate temperature fluctuations in remote or under-resourced areas. These enhancements in formulation and delivery are essential in bridging the accessibility gap between developed and developing nations.
Where Is Demand Concentrated and What Are the Emerging Use Cases?
The demand for IPV is highest in regions transitioning away from OPV, particularly in middle-income countries supported by the Global Polio Eradication Initiative (GPEI). With endemic transmission now limited to a few regions, surveillance systems are rapidly shifting to IPV-based containment strategies. Inactivated vaccines are also being stockpiled globally to manage outbreaks caused by vaccine-derived poliovirus strains, especially in areas with low immunization coverage. In high-income nations, IPV is a mainstay in routine childhood immunization, often delivered in combination vaccines to simplify administration.Inactivated rabies vaccines show particularly strong uptake in Asia-Pacific and Sub-Saharan Africa, where the disease burden is high. Governments and global health organizations are working to expand access to post-exposure prophylaxis (PEP), with improved availability in rural health centers and emergency departments. There is also a growing focus on pre-exposure vaccination for high-risk groups such as veterinarians, animal handlers, travelers, and laboratory personnel. The use of ID regimens has been critical in enabling mass vaccination campaigns during rabies outbreaks, particularly in India, Thailand, and the Philippines. Additionally, international travel protocols are influencing demand for both polio and rabies vaccines, especially in travelers visiting endemic regions.
The Growth in the Inactivated Polio and Rabies Vaccines Market Is Driven by Several Factors…
It is driven primarily by the phasing out of oral polio vaccines in favor of inactivated alternatives as part of global eradication initiatives, coupled with increased national investments in universal IPV immunization programs. The expansion of Gavi-supported immunization infrastructure and the integration of IPV into combination pediatric vaccines have significantly broadened the user base and streamlined logistics. In parallel, the rise in reported cases of rabies and limited access to timely post-exposure treatment in endemic countries are fueling sustained demand for inactivated rabies vaccines, particularly through intradermal, cost-effective administration.Another growth lever is the surge in global travel, migration, and awareness of travel-related infectious risks, which has increased the uptake of pre-exposure vaccination. Technological improvements in vaccine production and thermostability, combined with decentralization of manufacturing capabilities, are helping reduce costs and improve regional access, especially across low- and middle-income countries. Furthermore, national and international policy mandates, such as WHO prequalification requirements and regulatory fast-tracking for inactivated vaccines, are boosting procurement by public health authorities. The emergence of public-private partnerships and donor funding to support stockpiling and emergency response capacity for both diseases adds an additional layer of support to the market's continued expansion.
Report Scope
The report analyzes the Inactivated Polio and Rabies Vaccines market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Vaccine (Inactivated Polio Vaccines, Inactivated Rabies Vaccines); Inactivation Method (Solvent Detergent Method, Radiation Method, pH Concentration, Heat Inactivation, Other Inactivation Methods); Age Group (Pediatrics, Adults); By Distribution Channel (Government, Private).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Inactivated Polio Vaccines segment, which is expected to reach US$971.3 Million by 2030 with a CAGR of a 2.8%. The Inactivated Rabies Vaccines segment is also set to grow at 4.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $323.5 Million in 2024, and China, forecasted to grow at an impressive 6.4% CAGR to reach $288.8 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Inactivated Polio and Rabies Vaccines Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Inactivated Polio and Rabies Vaccines Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Inactivated Polio and Rabies Vaccines Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Angel Medical Systems, Inc., Biotronik SE & Co. KG, Boston Scientific Corporation, Cardiac Insight, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Inactivated Polio and Rabies Vaccines market report include:
- Bavarian Nordic A/S
- Bharat Biotech International Ltd.
- Biological E Limited
- BIO-MED Pvt. Ltd.
- Boehringer Ingelheim International GmbH
- Cadila Healthcare Limited (Zydus)
- CanSino Biologics Inc.
- Chiron Behring Vaccines Pvt. Ltd.
- CSL Limited
- Daiichi Sankyo Company, Limited
- Elanco Animal Health Inc.
- GlaxoSmithKline plc
- Indian Immunologicals Ltd.
- Johnson & Johnson Services Inc.
- Kedrion Biopharma Inc.
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi Pasteur
- Serum Institute of India Pvt. Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Bavarian Nordic A/S
- Bharat Biotech International Ltd.
- Biological E Limited
- BIO-MED Pvt. Ltd.
- Boehringer Ingelheim International GmbH
- Cadila Healthcare Limited (Zydus)
- CanSino Biologics Inc.
- Chiron Behring Vaccines Pvt. Ltd.
- CSL Limited
- Daiichi Sankyo Company, Limited
- Elanco Animal Health Inc.
- GlaxoSmithKline plc
- Indian Immunologicals Ltd.
- Johnson & Johnson Services Inc.
- Kedrion Biopharma Inc.
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi Pasteur
- Serum Institute of India Pvt. Ltd.
Table Information
| Report Attribute | Details |
|---|---|
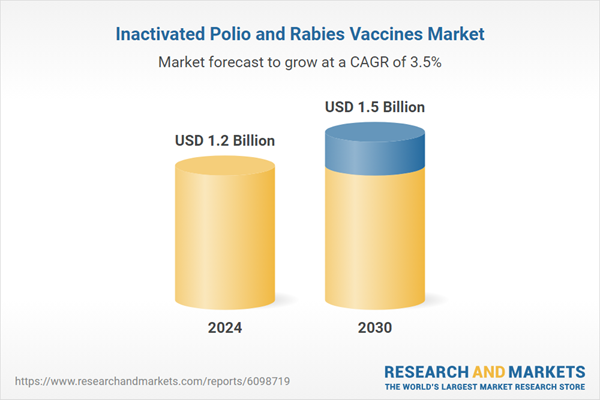
| No. of Pages | 470 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 1.2 Billion |
| Forecasted Market Value ( USD | $ 1.5 Billion |
| Compound Annual Growth Rate | 3.5% |
| Regions Covered | Global |