Global Organ Transplant Rejection Medications Market - Key Trends & Drivers Summarized
Why Is Immunosuppressive Therapy Central to the Success and Longevity of Organ Transplantation?
Organ transplant rejection medications - also known as immunosuppressive therapies - are essential for preventing the host immune system from attacking and destroying the transplanted organ. These medications form the backbone of post-transplant care and are critical to ensuring graft survival, minimizing complications, and improving patient quality of life. They are used immediately after transplantation (induction phase), throughout the maintenance period, and during acute rejection episodes to modulate the immune response.The global rise in solid organ transplants - covering kidneys, livers, hearts, lungs, and pancreases - is driving demand for reliable and safe immunosuppressive regimens. With survival rates improving due to surgical advancements and post-transplant monitoring, the long-term management of immune response has become a key therapeutic priority. The complexity of immune signaling pathways involved in allograft rejection requires multi-drug approaches, often combining calcineurin inhibitors (e.g., tacrolimus), mTOR inhibitors (e.g., sirolimus), corticosteroids, and antiproliferative agents like mycophenolate mofetil. Continuous innovation in these classes is enabling better tolerance, fewer infections, and reduced chronic toxicity.
How Are New Therapeutic Targets and Formulations Improving Rejection Management and Patient Outcomes?
Recent advancements in immunology and molecular pharmacology are leading to the development of novel immunosuppressive agents with more selective mechanisms of action and improved side effect profiles. Co-stimulation blockers (e.g., belatacept) and monoclonal antibodies (e.g., basiliximab) are offering targeted inhibition of T-cell activation pathways without the nephrotoxicity associated with traditional calcineurin inhibitors. These agents are particularly useful in renal transplant recipients and patients with comorbidities.Formulation improvements - such as extended-release tablets, enteric-coated capsules, and nano-carrier-based drug delivery - are enhancing medication adherence and pharmacokinetic stability. Therapeutic drug monitoring (TDM) is becoming more precise through point-of-care assays and pharmacogenomic profiling, allowing clinicians to individualize dosing and reduce rejection risk. Research is also underway to develop tolerance-inducing regimens that may one day eliminate the need for lifelong immunosuppression. These innovations are significantly reshaping the post-transplant medication landscape and improving both graft survival and patient quality of life.
Which Patient Populations and Regional Health Systems Are Driving Demand for Anti-Rejection Medications?
Kidney transplant recipients represent the largest segment of immunosuppressive therapy users, followed by liver and heart transplant patients. Pediatric and geriatric transplant populations require specialized dosing and monitoring strategies, due to differences in metabolism and immune system behavior. Patients undergoing re-transplantation or those with high panel reactive antibodies (PRA) often require more potent or multi-line regimens to prevent rejection and graft failure.The U.S. leads global adoption due to its high transplant volume, advanced donor-matching systems, and inclusion of immunosuppressants in public and private insurance plans. Europe maintains a strong position through coordinated transplant networks and centralized post-transplant care. Asia-Pacific is witnessing rapid growth, particularly in India, China, and Japan, where organ transplant programs are scaling up and immunosuppressant affordability is improving. Emerging markets in Latin America and the Middle East are expanding access through public health financing and regional partnerships with global pharma companies.
What Is Fueling Long-Term Growth and Strategic Innovation in the Organ Transplant Rejection Medications Market?
The growth in the organ transplant rejection medications market is driven by the increasing success rate and accessibility of transplant procedures, longer post-transplant survival, and rising organ donation rates globally. Lifelong reliance on immunosuppressive medications, coupled with a growing transplant-eligible patient population, ensures recurring demand and market stability. Additionally, innovations in precision dosing, targeted therapies, and biosimilar development are broadening treatment options while improving cost-effectiveness.Pharmaceutical companies are exploring next-generation biologics, T-regulatory cell therapy, and gene-editing strategies to induce immune tolerance and reduce long-term drug dependency. Strategic collaborations between transplant centers, biotech firms, and academic institutions are fostering accelerated clinical trials and real-world data generation. Regulatory support for fast-tracking therapies that reduce chronic rejection and improve long-term graft function is further encouraging innovation. As the global transplant ecosystem evolves toward personalized, sustainable, and high-outcome protocols, the market for rejection medications will continue to expand and diversify.
Report Scope
The report analyzes the Organ Transplant Rejection Medications market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Drug Class (Antibodies, Antimetabolites, Steroids, Other Drug Classes); Transplant Type (Kidney Transplant, Liver Transplant, Heart Transplant, Lung Transplant, Other Transplant Types); Distribution Channel (Hospitals Pharmacies Distribution Channel, Retail Pharmacies Distribution Channel, Online Pharmacies Distribution Channel).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Antibodies segment, which is expected to reach US$2.7 Billion by 2030 with a CAGR of a 1.7%. The Antimetabolites segment is also set to grow at 3.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.4 Billion in 2024, and China, forecasted to grow at an impressive 4.7% CAGR to reach $1.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Organ Transplant Rejection Medications Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Organ Transplant Rejection Medications Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Organ Transplant Rejection Medications Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AbbVie Inc., Aenova Group, Alcami Corporation, Almac Group, Amgen Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Organ Transplant Rejection Medications market report include:
- AbbVie Inc.
- Accord Healthcare Ltd.
- Astellas Pharma Inc.
- Biocon Limited
- Bristol-Myers Squibb Company
- Dr. Reddy’s Laboratories Ltd.
- F. Hoffmann-La Roche Ltd.
- Genzyme Corporation
- GlaxoSmithKline plc
- Glenmark Pharmaceuticals Ltd.
- Hansa Biopharma AB
- Hikma Pharmaceuticals plc
- Mylan Laboratories Inc.
- Novartis AG
- Panacea Biotec Ltd.
- Pfizer Inc.
- Sanofi S.A.
- Teva Pharmaceutical Industries Ltd.
- Veloxis Pharmaceuticals A/S
- Viatris Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Accord Healthcare Ltd.
- Astellas Pharma Inc.
- Biocon Limited
- Bristol-Myers Squibb Company
- Dr. Reddy’s Laboratories Ltd.
- F. Hoffmann-La Roche Ltd.
- Genzyme Corporation
- GlaxoSmithKline plc
- Glenmark Pharmaceuticals Ltd.
- Hansa Biopharma AB
- Hikma Pharmaceuticals plc
- Mylan Laboratories Inc.
- Novartis AG
- Panacea Biotec Ltd.
- Pfizer Inc.
- Sanofi S.A.
- Teva Pharmaceutical Industries Ltd.
- Veloxis Pharmaceuticals A/S
- Viatris Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 374 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
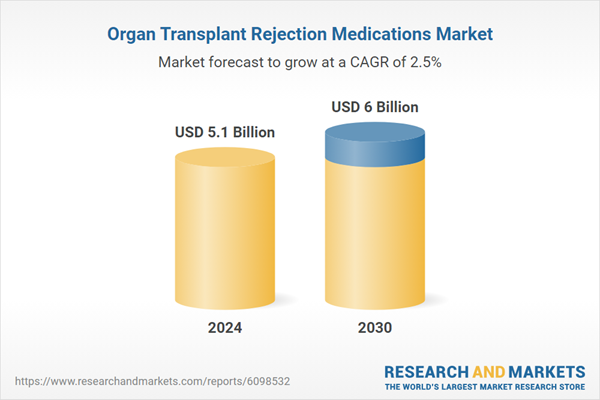
| Estimated Market Value ( USD | $ 5.1 Billion |
| Forecasted Market Value ( USD | $ 6 Billion |
| Compound Annual Growth Rate | 2.5% |
| Regions Covered | Global |