Global “Relapsed or Refractory Diffuse Large B-Cell Lymphoma (R/R DLBCL)” Market - Key Trends & Drivers Summarized
Why Is Relapsed or Refractory DLBCL a Persistent Challenge in Oncology?
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma, accounting for approximately 30% of all NHL cases worldwide. While first-line therapies such as R-CHOP (rituximab with chemotherapy) achieve remission in a significant number of patients, about 30-40% experience relapse or do not respond to initial treatment - classified as relapsed or refractory (R/R) DLBCL. This patient subgroup faces a poor prognosis, with limited curative options. The heterogeneity of the disease, often driven by genetic abnormalities like MYC, BCL2, and BCL6 rearrangements, complicates treatment strategies. Conventional salvage therapies such as high-dose chemotherapy followed by autologous stem cell transplant are often unsuitable due to age or comorbidities, leaving a therapeutic gap. The unmet clinical need in this space is immense, spurring ongoing research and innovation in both targeted and cellular immunotherapies.How Are Innovative Therapies Transforming the Outlook for R/R DLBCL Patients?
Breakthrough therapies are redefining the standard of care for R/R DLBCL and bringing renewed hope to patients who were previously considered incurable. Among the most impactful innovations are chimeric antigen receptor T-cell (CAR-T) therapies, including axicabtagene ciloleucel and tisagenlecleucel, which have shown substantial complete remission rates even in heavily pre-treated patients. These personalized cell-based therapies reprogram a patient's own immune cells to recognize and kill lymphoma cells. In parallel, antibody-drug conjugates (ADCs) like polatuzumab vedotin and bispecific antibodies such as glofitamab are emerging as viable off-the-shelf options with high efficacy and manageable toxicity profiles. Small molecule inhibitors targeting key pathways such as BTK, PI3K, and BCL2 are also under active investigation. The development of these therapies reflects a broader trend toward precision oncology, where treatments are increasingly tailored to the molecular profile of the patient's disease, transforming once-fatal relapses into manageable chronic conditions or potential cures.Which Healthcare and Market Dynamics Are Shaping the Adoption of These Therapies?
The R/R DLBCL treatment market is being shaped by a convergence of regulatory, healthcare system, and payer dynamics. Regulatory bodies such as the FDA and EMA have granted accelerated approvals and breakthrough designations to several advanced therapies, fast-tracking access and incentivizing further R&D. However, high treatment costs, especially for CAR-T therapies, have sparked debate over reimbursement models and accessibility. Payers are increasingly evaluating outcomes-based contracts and value-based care agreements to manage the financial risk of adopting these high-cost therapies. Healthcare providers are also investing in infrastructure and training for administering complex treatments like CAR-T, which require specialized centers and multidisciplinary teams. Meanwhile, pharmaceutical companies are developing next-generation CAR-Ts with reduced manufacturing times and off-the-shelf formats to improve scalability and reduce treatment delays. Patient advocacy groups and digital platforms are also playing a role by increasing awareness, supporting access programs, and connecting patients to clinical trials.The Growth in the Relapsed or Refractory DLBCL Market Is Driven by Breakthrough Immunotherapies, Precision Oncology Trends, and Infrastructure Expansion for Complex Biologics
The growth in the relapsed or refractory DLBCL market is driven by several interlinked factors. Chief among them is the rise of next-generation immunotherapies - particularly CAR-T cell therapies, ADCs, and bispecific antibodies - which are transforming survival outcomes for patients with limited options. Secondly, the adoption of precision medicine, enabled by genomic profiling and companion diagnostics, is allowing oncologists to identify high-risk DLBCL subsets and match them with targeted therapies. Thirdly, improvements in biopharmaceutical manufacturing, including automation and decentralized CAR-T production models, are enhancing the accessibility and scalability of advanced treatments. Fourthly, growing investments by hospitals and oncology centers in cell therapy units and specialized infusion facilities are making it easier to deliver complex biologics safely and efficiently. Additionally, supportive healthcare policies, fast-track approvals, and expanded clinical trial networks are helping bring innovative therapies to market faster. Lastly, patient demand for more durable and less toxic alternatives to traditional chemotherapy is pushing physicians and payers to adopt these novel therapies as part of an evolving standard of care.Report Scope
The report analyzes the Relapsed or Refractory Diffuse Large B Cell Lympho market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Drug Type (Monjuvi, XPOVIO, Polivy, Kymriah, Yescarta, Other Drug Types); Distribution Channel (Hospitals Pharmacies, Retail Pharmacies, Online Pharmacies).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Monjuvi segment, which is expected to reach US$456.4 Million by 2030 with a CAGR of a 1.7%. The XPOVIO segment is also set to grow at 3.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $408 Million in 2024, and China, forecasted to grow at an impressive 5.3% CAGR to reach $343 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Relapsed or Refractory Diffuse Large B Cell Lympho Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Relapsed or Refractory Diffuse Large B Cell Lympho Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Relapsed or Refractory Diffuse Large B Cell Lympho Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Air Clear LLC, Alliance Corporation, Anguil Environmental Systems, Inc., Babcock & Wilcox Enterprises Inc., Baolan EP Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Relapsed or Refractory Diffuse Large B Cell Lympho market report include:
- AbbVie Inc.
- Adaptive Biotechnologies Corp.
- ADC Therapeutics SA
- Bristol Myers Squibb
- Cellular Biomedicine Group Inc.
- Eagle Pharmaceuticals, Inc.
- Genmab A/S
- Gilead Sciences, Inc.
- Hoffmann-La Roche AG
- IMV Inc.
- Incyte Corporation
- Janssen Biotech, Inc.
- Karyopharm Therapeutics
- Merck & Co., Inc.
- MorphoSys U.S. Inc.
- Novartis AG
- Overland Pharmaceuticals
- Pfizer Inc.
- Regeneron Pharmaceuticals
- Takeda Pharmaceutical Company
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Adaptive Biotechnologies Corp.
- ADC Therapeutics SA
- Bristol Myers Squibb
- Cellular Biomedicine Group Inc.
- Eagle Pharmaceuticals, Inc.
- Genmab A/S
- Gilead Sciences, Inc.
- Hoffmann-La Roche AG
- IMV Inc.
- Incyte Corporation
- Janssen Biotech, Inc.
- Karyopharm Therapeutics
- Merck & Co., Inc.
- MorphoSys U.S. Inc.
- Novartis AG
- Overland Pharmaceuticals
- Pfizer Inc.
- Regeneron Pharmaceuticals
- Takeda Pharmaceutical Company
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 294 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
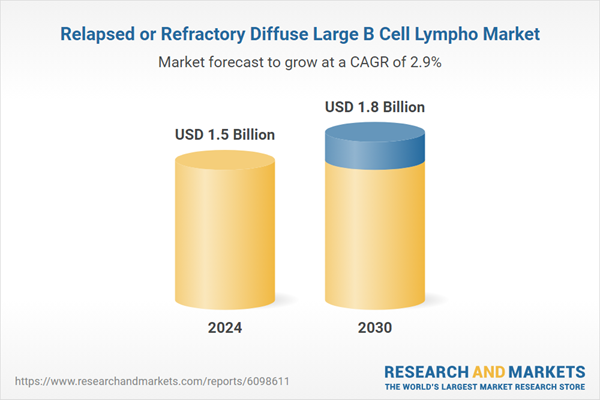
| Estimated Market Value ( USD | $ 1.5 Billion |
| Forecasted Market Value ( USD | $ 1.8 Billion |
| Compound Annual Growth Rate | 2.9% |
| Regions Covered | Global |