Global Cyproterone Acetate Market - Key Trends & Drivers Summarized
Why Is Cyproterone Acetate Becoming Central to Hormone-Dependent Therapy Across Medical Disciplines?
Cyproterone acetate (CPA), a synthetic steroidal anti-androgen and progestin, is a cornerstone pharmacological agent in the treatment of hormone-sensitive conditions such as prostate cancer, hirsutism, severe acne, androgenetic alopecia, and hypersexuality disorders. By inhibiting androgen receptors and suppressing luteinizing hormone (LH) secretion, CPA effectively blocks the biological actions of testosterone and dihydrotestosterone (DHT), making it a key agent in both feminizing hormone therapy and androgen-suppression regimens.Its dual action - anti-androgenic and progestogenic - enables it to reduce androgen production at both peripheral and central levels, offering therapeutic benefits in dermatology, urology, oncology, and gender-affirming care. As hormonal imbalances and endocrine disorders gain increasing recognition in both clinical and lifestyle contexts, cyproterone acetate is emerging as a first-line option in integrated hormone-modulating treatment protocols.
What Formulation Innovations and Therapeutic Trends Are Expanding CPA Usage?
Pharmaceutical innovations are expanding the formulation versatility and therapeutic reach of CPA. Available in oral tablets, injectables, and combination formulations (often with ethinylestradiol), CPA is being increasingly tailored for condition-specific dosing regimens. In the dermatological space, CPA is prescribed in low-dose combinations for women suffering from acne or hirsutism, often as part of oral contraceptive therapies. These combinations help regulate sebaceous gland activity and hair follicle sensitivity to androgens, with extended benefits in polycystic ovary syndrome (PCOS) management.In oncology, higher-dose CPA is used to treat advanced prostate cancer and mitigate tumor flare associated with initial luteinizing hormone-releasing hormone (LHRH) agonist therapy. It also serves as an effective chemical castration agent in patients requiring testosterone suppression. Transgender healthcare is another emerging application, where CPA plays a pivotal role in male-to-female hormone therapy regimens. Its cost-effectiveness and familiarity in clinical practice make it a preferred option in both public and private gender care programs.
Extended-release injectable formulations and research into transdermal delivery mechanisms are being explored to improve patient compliance and reduce hepatic metabolism. Pharmacovigilance data and real-world evidence are informing dosage refinements aimed at minimizing long-term side effects such as hepatotoxicity, mood changes, or thromboembolic risks.
Who Are the Primary End-Users and How Are Regional Treatment Patterns Influencing Market Demand?
The main end-users of cyproterone acetate include hospitals, dermatology clinics, oncology centers, gender clinics, and retail pharmacies. Dermatologists use CPA-based contraceptives for managing hormonal acne and hirsutism in women. Oncologists rely on high-dose CPA in prostate cancer management, particularly in countries where cost constraints or access to newer anti-androgens like enzalutamide or abiraterone limit treatment options.Gender clinics and endocrinologists increasingly prescribe CPA as part of feminizing hormone therapy protocols, often in combination with estradiol. Urologists also use CPA in managing benign prostatic hyperplasia and libido suppression in specific patient populations. In the psychiatric domain, CPA has been trialed for compulsive sexual behavior management in select cases.
Geographically, Europe leads in CPA adoption due to earlier regulatory approvals, strong reimbursement frameworks, and wider therapeutic recognition. Germany, France, and Eastern European countries exhibit high CPA usage, especially in dermatology and oncology. In Asia-Pacific, rising awareness of PCOS, expanding transgender healthcare services, and increasing prevalence of hormone-driven cancers are driving market penetration in India, Thailand, and South Korea. Latin America and the Middle East are witnessing growing demand through dermatological and contraceptive applications, though regulatory variability persists.
What Is Driving the Growth and Diversification of the Cyproterone Acetate Market?
The growth in the cyproterone acetate market is driven by increasing incidences of hormone-dependent conditions, the rise of personalized hormone therapy, and the expansion of gender-affirming healthcare services. As hormonal disorders such as PCOS, hyperandrogenism, and acne impact a growing female population, especially in urban and adolescent demographics, CPA-based therapies are gaining clinical prominence.The growth in prostate cancer incidence, particularly in aging male populations across developed and developing countries, is another major driver. CPA's affordability, oral bioavailability, and proven efficacy are sustaining its role in oncology despite newer agents. Meanwhile, increasing social and institutional support for transgender healthcare is catalyzing broader CPA adoption in hormone transition protocols.
Regulatory stability in key markets, availability in both generic and branded formats, and inclusion in national essential medicines lists are supporting market accessibility. However, long-term safety monitoring and pharmacovigilance remain essential to ensure continued clinical trust. With rising cross-specialty utilization, hormone modulation becoming mainstream, and therapeutic diversification accelerating, the global cyproterone acetate market is poised for sustained, medically driven expansion.
Report Scope
The report analyzes the Cyproterone Acetate market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Formulation (Tablets, Injectables, Topical Preparations); Application (Hormonal Replacement Therapy, Contraception, Acne Treatment, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Tablets Formulation segment, which is expected to reach US$253.2 Million by 2030 with a CAGR of a 4.5%. The Injectables Formulation segment is also set to grow at 2.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $86.5 Million in 2024, and China, forecasted to grow at an impressive 7% CAGR to reach $80.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Cyproterone Acetate Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Cyproterone Acetate Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Cyproterone Acetate Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Akzo Nobel N.V., BASF SE, Chevron Phillips Chemical Company LLC, China National Petroleum Corporation (CNPC), Clariant AG and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Cyproterone Acetate market report include:
- Axplora
- Bayer AG
- Cipla
- Curia
- Dr. Reddy's Laboratories
- Glenmark Pharmaceuticals
- Hubei Gedian Humanwell Pharmaceutical
- Jiangsu Grand Xianle Pharmaceutical Co. Ltd.
- Lupin Limited
- Newchem S.p.A.
- Sicor de México
- Swati Spentose
- TAPI Technology & API Services
- Temad Co.
- Teva Pharmaceutical Industries
- Torrent Pharmaceuticals Ltd.
- Unipex
- Viatris (formerly Mylan)
- Zhejiang Xianju Pharmaceutical Co. Ltd.
- Zydus Cadila (Cadila Healthcare Ltd.)
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Axplora
- Bayer AG
- Cipla
- Curia
- Dr. Reddy's Laboratories
- Glenmark Pharmaceuticals
- Hubei Gedian Humanwell Pharmaceutical
- Jiangsu Grand Xianle Pharmaceutical Co. Ltd.
- Lupin Limited
- Newchem S.p.A.
- Sicor de México
- Swati Spentose
- TAPI Technology & API Services
- Temad Co.
- Teva Pharmaceutical Industries
- Torrent Pharmaceuticals Ltd.
- Unipex
- Viatris (formerly Mylan)
- Zhejiang Xianju Pharmaceutical Co. Ltd.
- Zydus Cadila (Cadila Healthcare Ltd.)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 287 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
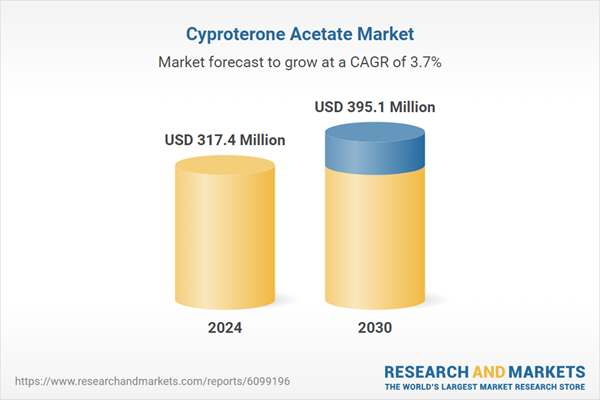
| Estimated Market Value ( USD | $ 317.4 Million |
| Forecasted Market Value ( USD | $ 395.1 Million |
| Compound Annual Growth Rate | 3.7% |
| Regions Covered | Global |