Global Anti-Epileptic Drugs for Pediatrics Market - Key Trends & Drivers Summarized
Why Are Pediatric Anti-Epileptic Drugs Gaining Greater Clinical and Regulatory Attention Worldwide?
The market for anti-epileptic drugs (AEDs) targeting pediatric populations is expanding rapidly due to the rising global burden of childhood epilepsy, increased focus on early intervention, and advances in precision neurology. Pediatric epilepsy - characterized by recurrent, unprovoked seizures - is one of the most common chronic neurological disorders in children, with onset frequently occurring in infancy or early childhood. The complex etiology of pediatric epilepsy, ranging from genetic mutations and neurodevelopmental syndromes to perinatal brain injury and metabolic disorders, necessitates age-appropriate, efficacious, and tolerable drug therapies. Conventional adult-centric AED regimens often pose challenges in pediatric use due to differences in pharmacokinetics, metabolism, and side effect profiles. As a result, there is growing regulatory and clinical emphasis on developing pediatric-specific formulations, dosing protocols, and indication extensions for approved molecules.Organizations such as the FDA and EMA are encouraging pharmaceutical companies to conduct pediatric trials under pediatric investigation plans (PIPs) and pediatric research equity acts (PREA), with incentives for label expansions and orphan drug designations. This regulatory momentum is driving formulation innovation in chewable tablets, suspensions, mini-tablets, and orally disintegrating dosage forms that accommodate pediatric swallowing and dosage titration requirements. At the same time, increasing access to neuroimaging, genetic screening, and electroencephalography (EEG) in emerging markets is facilitating earlier diagnosis, improving seizure classification, and driving demand for targeted pharmacologic interventions. These factors are repositioning pediatric epilepsy as a high-priority neurology segment for pharmaceutical innovation, public health planning, and caregiver support systems.
How Are Formulation Science and Drug Pipeline Innovation Enhancing Pediatric Treatment Outcomes?
Scientific advancements in formulation science and the clinical pipeline are reshaping the pediatric AED landscape, addressing long-standing challenges related to drug tolerability, polytherapy, and pharmacoresistance. Newer-generation AEDs - such as levetiracetam, oxcarbazepine, lamotrigine, topiramate, and brivaracetam - offer improved safety profiles, broader spectrum activity, and reduced sedative or cognitive side effects compared to older agents like phenobarbital and phenytoin. These drugs are increasingly available in pediatric-specific formats, including oral liquids, sprinkles, and dispersible tablets, which enable precise dosing based on body weight and developmental stage. Moreover, fixed-dose combinations (FDCs) and extended-release (ER) formulations are being developed to simplify dosing schedules, enhance adherence, and reduce seizure breakthrough risks during night-time or school hours.On the pipeline front, pharmaceutical research is focusing on rare and genetically defined pediatric epilepsies such as Dravet syndrome, Lennox-Gastaut syndrome (LGS), and tuberous sclerosis complex (TSC). Targeted therapies such as cannabidiol (CBD), stiripentol, fenfluramine, and everolimus are gaining approval or fast-track designations due to their role in seizure control where conventional AEDs fail. Novel mechanisms - such as selective sodium channel blockers, GABA modulators, and gene-based therapies - are under investigation to address drug-resistant epilepsy (DRE) and reduce long-term neurodevelopmental impact. The integration of pharmacogenetics into pediatric AED selection is also emerging, allowing clinicians to tailor therapy based on metabolic profile and seizure phenotype. These innovations are not only improving seizure control but also minimizing adverse developmental effects, a crucial consideration in pediatric neurologic care.
Where Is Market Demand Intensifying and Which Stakeholders Are Driving Adoption of Pediatric AEDs?
Demand for pediatric anti-epileptic drugs is intensifying across both developed and emerging markets, driven by increasing epilepsy diagnosis rates, growing access to pediatric neurology services, and heightened caregiver awareness. North America and Western Europe lead in terms of regulatory approvals, clinical trial activity, and early adoption of novel AEDs. The United States, in particular, has seen growing prescription volumes for pediatric-approved formulations of levetiracetam, lamotrigine, and cannabidiol-based therapies, aided by supportive reimbursement structures and caregiver advocacy. Europe's emphasis on orphan drug pathways and national healthcare coverage is similarly facilitating access to newer treatments for syndromic epilepsies.In Asia-Pacific and Latin America, improvements in healthcare infrastructure, public-private partnerships, and pediatric specialty care are expanding diagnosis and treatment access. Countries such as India, China, and Brazil are seeing increased uptake of oral suspension AEDs and low-cost generics, particularly through national health insurance schemes and child health programs. Hospital-based pediatric neurologists and epilepsy centers are playing a pivotal role in prescription decision-making, while general pediatricians are increasingly being trained in early seizure identification and AED management. The role of caregiver networks, epilepsy foundations, and school-based awareness campaigns is also growing, pushing for early treatment initiation, improved medication adherence, and better quality of life for children with epilepsy. These converging stakeholder efforts are catalyzing market growth while narrowing treatment gaps in underserved regions.
What Is Driving the Global Growth of the Pediatric Anti-Epileptic Drugs Market?
The growth in the pediatric anti-epileptic drugs market is driven by several factors, including increasing prevalence of childhood epilepsy, expanded clinical guidelines for early treatment, and the launch of age-adapted drug formulations with better safety and efficacy profiles. A critical growth driver is the rising awareness among parents, pediatricians, and neurologists about the long-term developmental consequences of uncontrolled seizures, prompting earlier diagnosis and therapy initiation. Advances in neurodiagnostic tools, genomic testing, and electronic health integration are also enabling personalized treatment selection and real-time monitoring of drug response.Regulatory incentives for pediatric drug development, orphan designations for rare epilepsy syndromes, and fast-track approvals for novel AEDs are fueling innovation pipelines and accelerating time-to-market. Simultaneously, collaborations between pharmaceutical companies, academic centers, and epilepsy advocacy groups are strengthening research funding, trial recruitment, and caregiver education. The emergence of biosimilars and regionally manufactured generics is enhancing affordability and access, particularly in lower-income countries. As pediatric neurology evolves toward precision therapeutics and holistic seizure management, a pressing question arises: Can pediatric anti-epileptic drug development keep pace with the clinical complexity and lifelong care needs of children living with epilepsy worldwide?
Report Scope
The report analyzes the Anti-Epileptic Drugs for Pediatrics market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Drug Type (First Generation Anti-epileptic Drugs, Second Generation Anti-epileptic Drugs, Third Generation Anti-epileptic Drugs); Administration Route (Oral, Injectable, Other Administration Routes); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Some of the 34 companies featured in this Anti-Epileptic Drugs for Pediatrics market report include -
- Abbott Laboratories
- AbbVie Inc.
- Alexza Pharmaceuticals, Inc.
- Alkem Laboratories Ltd.
- AstraZeneca plc
- Bausch Health Companies Inc.
- Bial - Portela & C.ª, S.A.
- Cephalon, Inc.
- Dr. Reddy's Laboratories Ltd.
- Eisai Co., Ltd.
- GlaxoSmithKline plc (GSK)
- GW Pharmaceuticals plc
- H. Lundbeck A/S
- Insys Therapeutics, Inc.
- Jazz Pharmaceuticals plc
- Johnson & Johnson
- Marinus Pharmaceuticals, Inc.
- Mylan N.V.
- Novartis AG
- Ovid Therapeutics Inc.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the First Generation Anti-epileptic Drugs segment, which is expected to reach US$814.9 Million by 2030 with a CAGR of a 5%. The Second Generation Anti-epileptic Drugs segment is also set to grow at 6.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $294.3 Million in 2024, and China, forecasted to grow at an impressive 8.9% CAGR to reach $302.9 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Anti-Epileptic Drugs for Pediatrics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Anti-Epileptic Drugs for Pediatrics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Anti-Epileptic Drugs for Pediatrics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abcam plc, AbCellera Biologics Inc., Ablexis, LLC, Adimab LLC, Akeso Biopharma and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 34 Featured):
- Abbott Laboratories
- AbbVie Inc.
- Alexza Pharmaceuticals, Inc.
- Alkem Laboratories Ltd.
- AstraZeneca plc
- Bausch Health Companies Inc.
- Bial - Portela & C.ª, S.A.
- Cephalon, Inc.
- Dr. Reddy's Laboratories Ltd.
- Eisai Co., Ltd.
- GlaxoSmithKline plc (GSK)
- GW Pharmaceuticals plc
- H. Lundbeck A/S
- Insys Therapeutics, Inc.
- Jazz Pharmaceuticals plc
- Johnson & Johnson
- Marinus Pharmaceuticals, Inc.
- Mylan N.V.
- Novartis AG
- Ovid Therapeutics Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- AbbVie Inc.
- Alexza Pharmaceuticals, Inc.
- Alkem Laboratories Ltd.
- AstraZeneca plc
- Bausch Health Companies Inc.
- Bial - Portela & C.ª, S.A.
- Cephalon, Inc.
- Dr. Reddy's Laboratories Ltd.
- Eisai Co., Ltd.
- GlaxoSmithKline plc (GSK)
- GW Pharmaceuticals plc
- H. Lundbeck A/S
- Insys Therapeutics, Inc.
- Jazz Pharmaceuticals plc
- Johnson & Johnson
- Marinus Pharmaceuticals, Inc.
- Mylan N.V.
- Novartis AG
- Ovid Therapeutics Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 367 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
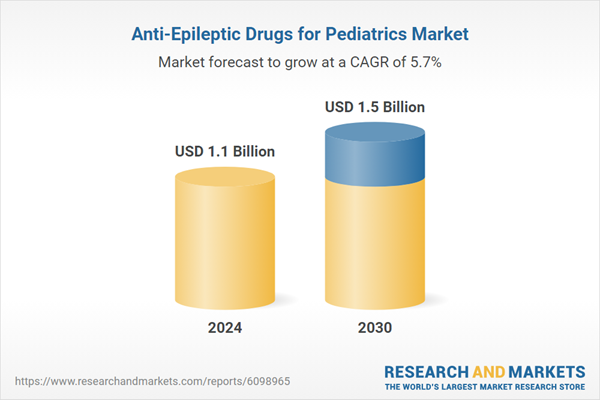
| Estimated Market Value ( USD | $ 1.1 Billion |
| Forecasted Market Value ( USD | $ 1.5 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |