Global Aplastic Anemia Market - Key Trends & Drivers Summarized
Why Is Aplastic Anemia Receiving Renewed Focus in Rare Disease Therapeutics?
Aplastic anemia - a rare and life-threatening bone marrow failure disorder characterized by pancytopenia and hypocellular marrow - is drawing increasing attention in the global healthcare ecosystem due to advances in diagnosis, growing awareness among hematologists, and the expanding pipeline of targeted therapies. The disease, which can be acquired or inherited, leads to a deficiency in red cells, white cells, and platelets, resulting in severe fatigue, infections, and bleeding complications. Historically managed with broad-spectrum immunosuppressants and bone marrow transplantation, treatment options were limited by donor availability, relapse risk, and long-term toxicity. However, improved molecular understanding of pathogenesis - especially the role of immune-mediated marrow suppression and telomere biology - is driving development of novel therapeutic strategies that aim to enhance hematopoiesis, reduce immune dysregulation, and improve survival rates.The increasing recognition of aplastic anemia as part of the broader spectrum of bone marrow failure syndromes is prompting greater investment in specialized care centers, early screening protocols, and precision medicine approaches. Regulatory frameworks supporting orphan drug designations and rare disease reimbursement are further incentivizing pharmaceutical research in this space. As patients with aplastic anemia often present with overlapping features of paroxysmal nocturnal hemoglobinuria (PNH) or myelodysplastic syndromes (MDS), there is growing emphasis on comprehensive diagnostics and risk stratification to enable individualized therapy. With unmet clinical needs remaining high and survival still suboptimal in refractory or relapsed cases, aplastic anemia is steadily emerging as a priority focus area within rare hematology portfolios.
How Are Therapeutic Innovations and Transplant Strategies Advancing Treatment Outcomes?
Therapeutic advancements in aplastic anemia are expanding beyond traditional immunosuppressive therapy (IST), with novel agents and combination regimens showing improved response rates and hematologic recovery. The standard first-line IST - comprising antithymocyte globulin (ATG) and cyclosporine A (CsA) - continues to be widely used, particularly for patients ineligible for stem cell transplantation. However, the addition of eltrombopag, a thrombopoietin receptor agonist, has significantly enhanced outcomes by stimulating multilineage hematopoiesis and improving overall response in both treatment-naïve and refractory patients. This triple-drug protocol is now considered a new standard of care in several guidelines, particularly for adult patients lacking matched sibling donors.In the curative setting, hematopoietic stem cell transplantation (HSCT) remains the gold standard for young, severe aplastic anemia patients with compatible donors. Innovations in conditioning regimens, graft-versus-host disease (GVHD) prophylaxis, and haploidentical transplantation are expanding the donor pool and reducing post-transplant complications. Research into telomerase activators, targeted complement inhibitors (in cases with overlapping PNH), and immune checkpoint modulation is also gaining momentum. Gene therapy is under preliminary exploration for inherited bone marrow failure syndromes that mimic aplastic anemia phenotypes. These therapeutic innovations are reinforcing a more nuanced, stage-adapted approach to treatment - balancing efficacy, toxicity, and quality of life - while moving closer to durable remission and cure in broader patient populations.
Where Is Demand for Aplastic Anemia Treatment Growing and Which Regions Are Seeing Clinical Progress?
Global demand for aplastic anemia treatment is expanding across North America, Europe, and Asia-Pacific, with variations in treatment access, donor availability, and diagnostic capabilities influencing regional market dynamics. The United States and Western Europe are at the forefront of clinical research, with advanced transplant infrastructure, greater uptake of eltrombopag-based regimens, and access to experimental therapies through clinical trials. Tertiary care centers and academic institutions in these regions are also leading collaborative registries and rare disease networks that facilitate real-world evidence collection and protocol optimization.Asia-Pacific - particularly China, India, and Japan - is witnessing significant growth in diagnosis and treatment due to higher disease prevalence, improved healthcare access, and government support for rare disease programs. China, in particular, has a large pool of aplastic anemia patients and is increasing local production of IST agents, stem cell therapies, and thrombopoietin agonists to meet growing demand. In India, while donor registry limitations persist, cost-effective IST regimens and expanding transplant capacity are improving outcomes in both pediatric and adult cohorts. Emerging markets in Latin America and the Middle East are gradually improving diagnostic timelines and treatment equity through international aid, centralized hematology programs, and partnerships with global health organizations. Across all regions, rising awareness and multidisciplinary care models are improving patient referral, follow-up, and access to lifesaving therapies.
What Is Driving the Global Growth of the Aplastic Anemia Therapeutics Market?
The growth in the aplastic anemia market is driven by several factors, including advances in immunotherapy and stem cell biology, expanding treatment access, and supportive policy frameworks for rare diseases. A key growth driver is the emergence of combination therapy approaches - particularly eltrombopag with IST - which are improving hematologic recovery and reducing early mortality. Regulatory designations such as orphan drug and breakthrough therapy status are expediting the development and approval of novel agents. Increased investment in hematologic research, precision diagnostics, and donor registry infrastructure is also strengthening treatment pathways and transplant readiness.Pharmaceutical innovation is being further supported by academic-industry collaborations, global patient registries, and outcome tracking systems that enable data-driven improvements in therapy selection. The growing role of digital health in managing long-term follow-up, medication adherence, and telemonitoring of cytopenias is enhancing continuity of care in chronic management. Public awareness campaigns and rare disease advocacy are helping reduce diagnostic delays and improve healthcare provider engagement. As therapeutic options diversify and survival improves, a critical question now shapes the future trajectory of this market: Can aplastic anemia care evolve from a reactive, donor-dependent model into a targeted, universally accessible paradigm of personalized hematologic restoration?
Report Scope
The report analyzes the Aplastic Anemia market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Disease Type (Acquired Aplastic Anemia, Inherited Aplastic Anemia); Treatment Type (Bone Marrow Transfusion, Blood Transfusion, Drug Therapy); Mode of Administration (Injectable, Oral, Other Mode of Administrations); End-Use (Hospitals, Specialty Clinics, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Some of the 43 companies featured in this Aplastic Anemia market report include -
- AbbVie Inc.
- Amgen Inc.
- Amyndas Pharmaceuticals S.A.
- Bayer AG
- BioLineRx Ltd.
- Bluebird Bio, Inc.
- Bristol-Myers Squibb Company
- Cadila Healthcare Limited (Zydus)
- Cellenkos Inc.
- CSL Limited
- Eisai Co., Ltd.
- Elixirgen Therapeutics, LLC
- F. Hoffmann-La Roche Ltd.
- Foresee Pharmaceuticals Co., Ltd.
- Gamida Cell Ltd.
- GlaxoSmithKline plc
- Hangzhou Zede Pharmaceutical Tech.
- Hemogenyx Pharmaceuticals plc
- Johnson & Johnson
- Kyowa Kirin Co., Ltd.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Acquired Aplastic Anemia segment, which is expected to reach US$5.9 Billion by 2030 with a CAGR of a 4%. The Inherited Aplastic Anemia segment is also set to grow at 1.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.8 Billion in 2024, and China, forecasted to grow at an impressive 6.4% CAGR to reach $1.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Aplastic Anemia Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Aplastic Anemia Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Aplastic Anemia Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Adyen, Affirm, Airwallex, Alloy, Basiq and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 43 Featured):
- AbbVie Inc.
- Amgen Inc.
- Amyndas Pharmaceuticals S.A.
- Bayer AG
- BioLineRx Ltd.
- Bluebird Bio, Inc.
- Bristol-Myers Squibb Company
- Cadila Healthcare Limited (Zydus)
- Cellenkos Inc.
- CSL Limited
- Eisai Co., Ltd.
- Elixirgen Therapeutics, LLC
- F. Hoffmann-La Roche Ltd.
- Foresee Pharmaceuticals Co., Ltd.
- Gamida Cell Ltd.
- GlaxoSmithKline plc
- Hangzhou Zede Pharmaceutical Tech.
- Hemogenyx Pharmaceuticals plc
- Johnson & Johnson
- Kyowa Kirin Co., Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Amgen Inc.
- Amyndas Pharmaceuticals S.A.
- Bayer AG
- BioLineRx Ltd.
- Bluebird Bio, Inc.
- Bristol-Myers Squibb Company
- Cadila Healthcare Limited (Zydus)
- Cellenkos Inc.
- CSL Limited
- Eisai Co., Ltd.
- Elixirgen Therapeutics, LLC
- F. Hoffmann-La Roche Ltd.
- Foresee Pharmaceuticals Co., Ltd.
- Gamida Cell Ltd.
- GlaxoSmithKline plc
- Hangzhou Zede Pharmaceutical Tech.
- Hemogenyx Pharmaceuticals plc
- Johnson & Johnson
- Kyowa Kirin Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 469 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
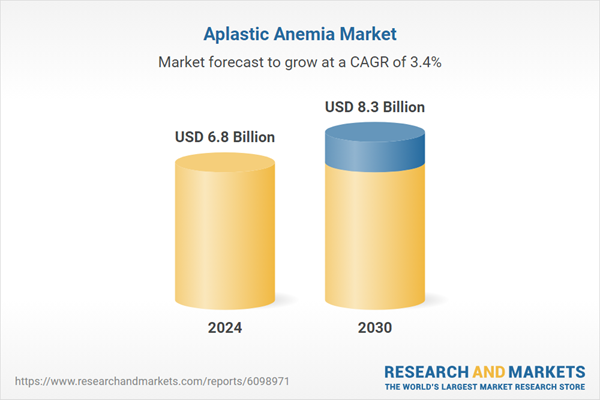
| Estimated Market Value ( USD | $ 6.8 Billion |
| Forecasted Market Value ( USD | $ 8.3 Billion |
| Compound Annual Growth Rate | 3.4% |
| Regions Covered | Global |