Global Auto-Retractable Safety Syringes Market - Key Trends & Drivers Summarized
Why Are Auto-Retractable Safety Syringes Gaining Critical Importance in Infection Control and Needle Stick Injury Prevention?
Auto-retractable safety syringes are rapidly emerging as essential tools in global healthcare systems, driven by the urgent need to mitigate needlestick injuries, cross-contamination, and the transmission of bloodborne pathogens such as HIV, hepatitis B, and hepatitis C. These syringes are engineered to automatically retract the needle into the barrel immediately after use, rendering reuse impossible and minimizing exposure risk for healthcare workers, patients, and waste handlers. With rising global awareness around healthcare worker safety and increased regulatory oversight, auto-retractable syringes are being positioned as a standard rather than optional injection device in both developed and developing regions.The global focus on safe injection practices, especially following large-scale immunization campaigns during the COVID-19 pandemic, has further accelerated the shift toward safety-engineered injection devices. International health bodies such as the WHO, UNICEF, and GAVI are actively advocating the use of auto-disable and retractable syringes in public health programs. In addition to improving occupational safety, these syringes play a crucial role in infection prevention and waste management, aligning with broader goals of healthcare quality, biosafety, and sustainability.
How Are Mechanical Design, Material Compatibility, and Regulatory Compliance Advancing Syringe Safety and Usability?
Technological advancements in auto-retractable syringe design are enhancing user ergonomics, device reliability, and manufacturing scalability. Most modern designs employ spring-based or plunger-activated retraction mechanisms that are intuitive and require no additional steps for activation. These systems are being optimized for minimal injection force, smooth fluid delivery, and clear actuation cues to support high compliance in fast-paced clinical environments. Single-handed operation and compatibility with standard aseptic techniques are central to ensuring widespread acceptance among healthcare providers.Material innovations - including medical-grade polypropylene barrels, silicone-coated rubber plungers, and stainless steel needles - ensure sterility, chemical compatibility, and safe disposal. Manufacturing processes are evolving to support automation, precision molding, and unit-level sterility assurance. Global regulatory requirements, including U.S. OSHA standards, EU Sharps Directive, and ISO 23908, are driving standardization and performance benchmarking across suppliers. As procurement decisions increasingly emphasize compliance, quality certification, and injury prevention efficacy, manufacturers are prioritizing product validation, usability studies, and risk-reduction data to meet tender and public sector demands.
Where Is Demand for Auto-Retractable Safety Syringes Expanding and Which Use Cases Are Driving Adoption?
Demand for auto-retractable safety syringes is expanding globally, with high uptake in North America and Europe due to stringent occupational health regulations and institutional protocols. In Asia-Pacific, Latin America, and Africa, adoption is gaining momentum through government-backed immunization drives, donor-funded public health programs, and increasing awareness among healthcare providers about sharps safety. Countries with large public healthcare systems or histories of unsafe injection practices are prioritizing the shift to auto-retractable syringes to curb iatrogenic infections and meet WHO-recommended best practices.Key use cases include routine immunizations, intravenous drug delivery, insulin administration, and high-volume vaccination campaigns - particularly in community health centers, field clinics, and mobile medical units. Hospitals, emergency care centers, and outpatient departments are adopting auto-retractable syringes to enhance safety in high-exposure settings such as trauma, infectious disease, and pediatrics. Additionally, NGOs and humanitarian organizations are integrating these syringes into medical kits for disaster relief, refugee health programs, and rural outreach, where protection against needlestick injury is critical under resource-limited conditions.
What Is Fueling the Global Growth of the Auto-Retractable Safety Syringes Market?
The global growth of the auto-retractable safety syringes market is driven by a combination of regulatory mandates, healthcare safety standards, and increasing healthcare access across emerging economies. The growing burden of needle-transmitted infections, combined with mounting legal and occupational liabilities from accidental needlesticks, is compelling healthcare institutions to transition from conventional to safety-engineered syringes. Policy support from global health organizations and procurement guidelines from donor agencies are further institutionalizing the adoption of auto-retractable formats in public sector healthcare delivery.Manufacturers are scaling up production capacity and expanding product portfolios to meet diverse clinical needs - from pediatric to large-volume syringes - while offering cost-effective variants tailored for bulk procurement. Procurement frameworks emphasizing lowest total cost of ownership (TCO), rather than just unit price, are also favoring auto-retractable designs that reduce downstream treatment costs from infection or injury. As the healthcare industry prioritizes biosafety, sustainability, and patient-centric care delivery, a strategic question defines the market's trajectory: Can auto-retractable safety syringes become the universal standard for injectable care delivery across all healthcare tiers - balancing cost, compliance, and clinical efficacy in a global push toward safer, smarter medical practices?
Report Scope
The report analyzes the Auto-Retractable Safety Syringes market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Capacity (0.5 ml Capacity, 1 ml Capacity, 3 ml Capacity, Other Capacities); Application (Intramuscular, Subcutaneous, Intravenous).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the 0.5 ml Capacity Syringes segment, which is expected to reach US$1.6 Billion by 2030 with a CAGR of a 1.9%. The 1 ml Capacity Syringes segment is also set to grow at 1.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $891.4 Million in 2024, and China, forecasted to grow at an impressive 4.3% CAGR to reach $708.4 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Auto-Retractable Safety Syringes Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Auto-Retractable Safety Syringes Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Auto-Retractable Safety Syringes Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Advansta Inc., Agfa-Gevaert N.V., Bio-Rad Laboratories, Inc., Carestream Health, Chemglass Life Sciences and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Auto-Retractable Safety Syringes market report include:
- AdvaCare Pharma
- Anhui JN Medical Device Co., Ltd.
- Axel Bio Corporation
- B. Braun Melsungen AG
- Baxter International Inc.
- BD (Becton, Dickinson and Company)
- Cardinal Health Inc.
- Changzhou Holinx Industries Co., Ltd.
- Changzhou Sunton Medical Technology Co.
- DMC Medical Limited
- Frontier Healthcare
- Gerresheimer AG
- Globe Medical Tech, Inc.
- Guangdong Haiou Medical Apparatus Co.
- Henke-Sass, Wolf GmbH
- Hindustan Syringes & Medical Devices Ltd.
- Jiangsu Jichun Medical Devices Co., Ltd.
- JMI Syringes & Medical Devices Ltd.
- Maidikang Medical Products (Shandong) Co., Ltd.
- Medefil, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AdvaCare Pharma
- Anhui JN Medical Device Co., Ltd.
- Axel Bio Corporation
- B. Braun Melsungen AG
- Baxter International Inc.
- BD (Becton, Dickinson and Company)
- Cardinal Health Inc.
- Changzhou Holinx Industries Co., Ltd.
- Changzhou Sunton Medical Technology Co.
- DMC Medical Limited
- Frontier Healthcare
- Gerresheimer AG
- Globe Medical Tech, Inc.
- Guangdong Haiou Medical Apparatus Co.
- Henke-Sass, Wolf GmbH
- Hindustan Syringes & Medical Devices Ltd.
- Jiangsu Jichun Medical Devices Co., Ltd.
- JMI Syringes & Medical Devices Ltd.
- Maidikang Medical Products (Shandong) Co., Ltd.
- Medefil, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 283 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
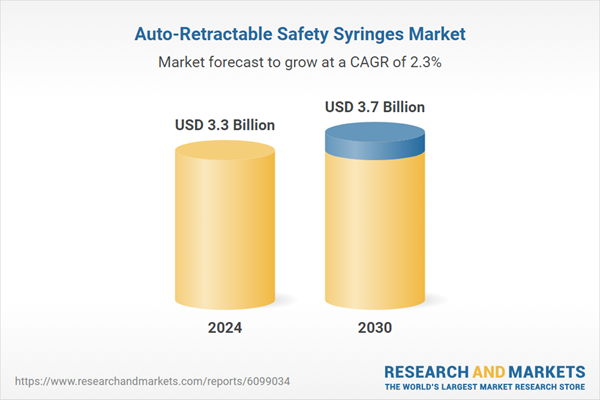
| Estimated Market Value ( USD | $ 3.3 Billion |
| Forecasted Market Value ( USD | $ 3.7 Billion |
| Compound Annual Growth Rate | 2.3% |
| Regions Covered | Global |