Global Bilirubin Meters Market - Key Trends & Drivers Summarized
Why Are Bilirubin Meters Gaining Clinical Importance in Neonatal and Hepatic Health Management?
Bilirubin meters are becoming increasingly essential in clinical diagnostics, particularly for early detection and monitoring of neonatal jaundice and liver-related disorders. These devices offer a rapid, non-invasive or minimally invasive means of measuring bilirubin concentration in blood or through transcutaneous screening - providing timely insight into liver function, hemolysis, and metabolic clearance. As neonatal hyperbilirubinemia remains one of the most common clinical conditions in newborns, early screening using bilirubin meters is critical to preventing complications such as kernicterus and bilirubin-induced neurologic dysfunction (BIND).In addition to neonatal care, bilirubin meters are being utilized in monitoring hepatic dysfunction, biliary obstruction, and hemolytic diseases in adults. The growing emphasis on point-of-care diagnostics, coupled with the global prioritization of early intervention in maternal and infant health programs, is driving adoption across hospitals, birthing centers, and outpatient pediatric care. As healthcare providers seek real-time bilirubin assessment tools that reduce turnaround times and improve patient throughput, these meters are gaining relevance as frontline diagnostic assets.
How Are Technology Enhancements and Workflow Integration Advancing Bilirubin Meter Performance?
Technological advancements in bilirubin meters are improving measurement accuracy, user convenience, and diagnostic consistency. Transcutaneous bilirubinometers now use advanced spectrophotometry and multi-wavelength optical sensors to detect bilirubin levels non-invasively, minimizing the need for blood draws in neonates. These devices are designed for repeatable measurements across different skin tones, gestational ages, and clinical conditions - supporting widespread adoption in neonatal ICUs and postnatal wards.Handheld, portable models with touchscreen interfaces, auto-calibration features, and wireless connectivity are facilitating integration into electronic health records (EHRs) and hospital information systems. Battery-operated designs with data logging capabilities enhance mobility and enable use in rural outreach programs, ambulatory settings, and home-based newborn monitoring. Manufacturers are also focusing on miniaturization, sensor precision, and dual-function models that combine bilirubin measurement with hemoglobin or oxygen saturation parameters - broadening their utility within pediatric and hepatic diagnostic workflows.
Where Is Demand for Bilirubin Meters Rising and Which Care Settings Are Driving Adoption?
Demand is strongest in developed markets such as North America and Europe, where universal newborn screening protocols and robust clinical infrastructure support early jaundice detection. Asia-Pacific is emerging as a high-growth region due to high birth rates, expanding neonatal care access, and public health initiatives targeting perinatal complications. Countries such as India, China, Indonesia, and Vietnam are witnessing increased procurement of bilirubin meters in both public and private healthcare facilities to reduce preventable infant morbidity and mortality.Primary adoption is concentrated in neonatal intensive care units (NICUs), maternity hospitals, and pediatric clinics. Community health programs and midwifery networks are also increasingly equipped with portable bilirubinometers for early screening during postnatal home visits. In adult medicine, hepatology clinics and liver transplant centers rely on lab-based bilirubin analyzers as part of liver function panel diagnostics. With global efforts intensifying around universal health coverage and rural health equity, decentralized access to accurate bilirubin monitoring is becoming a critical component of diagnostic capacity-building.
What Is Fueling the Global Growth of the Bilirubin Meters Market?
The global growth of the bilirubin meters market is being driven by rising neonatal care standards, the prioritization of maternal and child health, and growing awareness of the consequences of delayed jaundice detection. Technological innovation, regulatory support for non-invasive screening tools, and integration with mobile health platforms are making these devices more accessible and scalable across both high- and low-resource healthcare systems. Demand is also reinforced by the clinical shift toward real-time, bedside testing for faster diagnosis and intervention.Public-private partnerships, donations through global health organizations, and increased government procurement under infant health programs are accelerating market penetration in emerging economies. Manufacturers are expanding product lines with user-friendly, maintenance-light models designed for frontline health workers. As patient safety, early intervention, and diagnostic decentralization continue to shape healthcare priorities, a critical question emerges: Can the bilirubin meter market scale non-invasive, high-accuracy solutions fast enough to support universal newborn screening and broader hepatic health surveillance across global care ecosystems?
Report Scope
The report analyzes the Bilirubin Meters market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Benchtop, Transcutaneous); Indication (Jaundice, Hepatitis); Age Group (Neonatal, Adults); End-User (Hospitals & Nursing Homes, Clinics, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Benchtop Meters segment, which is expected to reach US$1.3 Billion by 2030 with a CAGR of a 7.9%. The Transcutaneous Meters segment is also set to grow at 4.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $341.3 Million in 2024, and China, forecasted to grow at an impressive 10.8% CAGR to reach $389.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Bilirubin Meters Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Bilirubin Meters Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Bilirubin Meters Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 3B Medical, Beijing Aeonmed Co., Ltd., Becton Dickinson (BD), BMC Medical Co., Ltd., Canta Medical Tech and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Bilirubin Meters market report include:
- Advanced Instruments, LLC
- Apel Co., Ltd.
- AVI Healthcare Pvt. Ltd.
- Danaher Corporation
- DAS Srl
- Drägerwerk AG & Co. KGaA
- F. Hoffmann-La Roche Ltd.
- Ginevri Srl
- GPC Medical Ltd.
- Hadleigh Health Technologies
- ICEN Technology Company Limited
- Koninklijke Philips N.V.
- Labtron Equipment Ltd.
- Mennen Medical Ltd.
- Micro Lab Instruments
- Mindray Bio-Medical Electronics Co., Ltd.
- Natus Medical Incorporated
- Ningbo David Medical Device Co., Ltd.
- Siemens Healthineers AG
- Xuzhou Kejian Hi-tech Co., Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Advanced Instruments, LLC
- Apel Co., Ltd.
- AVI Healthcare Pvt. Ltd.
- Danaher Corporation
- DAS Srl
- Drägerwerk AG & Co. KGaA
- F. Hoffmann-La Roche Ltd.
- Ginevri Srl
- GPC Medical Ltd.
- Hadleigh Health Technologies
- ICEN Technology Company Limited
- Koninklijke Philips N.V.
- Labtron Equipment Ltd.
- Mennen Medical Ltd.
- Micro Lab Instruments
- Mindray Bio-Medical Electronics Co., Ltd.
- Natus Medical Incorporated
- Ningbo David Medical Device Co., Ltd.
- Siemens Healthineers AG
- Xuzhou Kejian Hi-tech Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 454 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
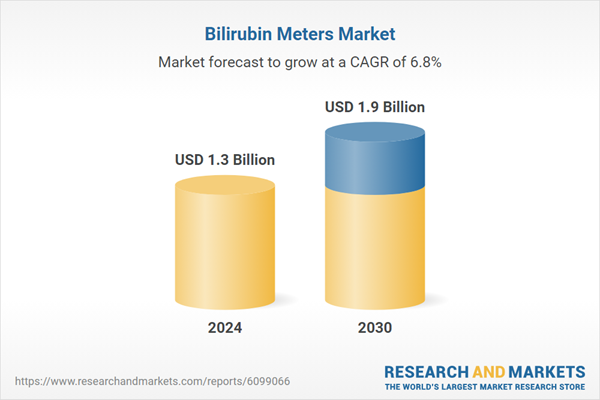
| Estimated Market Value ( USD | $ 1.3 Billion |
| Forecasted Market Value ( USD | $ 1.9 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |