Global Biosimilar Monoclonal Antibody Market - Key Trends & Drivers Summarized
Why Are Biosimilar Monoclonal Antibodies Becoming Pivotal in Expanding Access to Targeted Biologic Therapies?
Biosimilar monoclonal antibodies (mAbs) are gaining strategic importance in global healthcare systems due to their ability to replicate the efficacy, safety, and clinical performance of originator biologics at significantly lower costs. As biologics become central to the treatment of cancer, autoimmune diseases, and chronic inflammatory conditions, the expiration of patents on blockbuster mAbs has opened the market for biosimilar alternatives - addressing both cost containment imperatives and the need to widen patient access to advanced therapeutics.Unlike generic small molecules, biosimilar mAbs undergo rigorous analytical characterization and clinical validation to demonstrate high similarity without clinically meaningful differences. Their approval and commercialization are enabling healthcare providers to reduce biologics-related expenditure, expand formulary inclusion, and improve therapy access across public and private settings. In high-burden disease areas like rheumatoid arthritis, breast cancer, and colorectal cancer, biosimilar mAbs are transforming the economics of biologic care.
How Are Regulatory Pathways, Manufacturing Scale, and Clinical Confidence Accelerating Market Uptake?
Streamlined biosimilar regulatory frameworks - such as the EMA's well-established pathway, the FDA's 351(k) approval process, and WHO guidance - have matured to support the global rollout of biosimilar mAbs. These frameworks focus on extensive comparability exercises rather than full-scale efficacy trials, enabling faster and cost-efficient development. Regulatory convergence is also facilitating cross-market approvals and accelerating global launch timelines.Advancements in upstream cell-line engineering, downstream purification processes, and analytical platforms are improving biosimilar manufacturing precision, while reducing development variability and production costs. Simultaneously, post-marketing surveillance data and real-world evidence are reinforcing physician confidence in switching and initiating treatment with biosimilars. Provider education, stakeholder engagement, and payer incentives are also increasing prescriber alignment and institutional adoption across hospitals, oncology centers, and specialty clinics.
Where Is Demand for Biosimilar mAbs Expanding and Which Therapeutic Areas Are Leading Growth?
Europe remains the largest and most mature market for biosimilar monoclonal antibodies, supported by proactive healthcare policy, national biosimilar adoption targets, and tender-based procurement models. North America is witnessing accelerated uptake, particularly following the introduction of biosimilars for trastuzumab, bevacizumab, adalimumab, and rituximab. Asia-Pacific is emerging as a high-growth region with a strong manufacturing base, government-led cost-reduction programs, and rising biologics demand in countries such as India, South Korea, China, and Japan.Leading therapeutic segments include oncology - particularly breast, lung, and colorectal cancers - where trastuzumab, bevacizumab, and rituximab biosimilars are displacing originator therapies in first- and second-line protocols. In immunology, biosimilars for infliximab, adalimumab, and etanercept are being widely used for rheumatoid arthritis, psoriasis, and inflammatory bowel disease. Hospital-based treatment regimens, specialty pharmacy distribution, and payer-driven substitution policies are key drivers of market volume across these high-cost disease categories.
What Is Fueling the Global Growth of the Biosimilar Monoclonal Antibody Market?
The global growth of the biosimilar mAbs market is being fueled by the dual pressures of biologic drug affordability and therapeutic demand expansion. As healthcare systems seek sustainable models for managing chronic and complex diseases, biosimilars are offering competitive pricing with proven clinical equivalence. Payer incentives, supportive regulation, and improved switching protocols are facilitating institutional acceptance, while originator off-patent expirations continue to unlock new commercial opportunities.Strategic partnerships, co-development deals, and investment in large-scale biologics manufacturing facilities are supporting supply reliability and product differentiation. Market players are also engaging in targeted physician education, real-world outcome tracking, and multi-channel distribution to accelerate trust and uptake. As biosimilar mAbs become embedded in treatment algorithms and reimbursement schemes, a defining question shapes the next phase: Can the biosimilar industry maintain pricing competitiveness and manufacturing integrity - while scaling global access to high-cost biologic therapies without compromising safety, quality, or clinical outcomes?
Report Scope
The report analyzes the Biosimilar Monoclonal Antibody market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Adalimumab, Bevacizumab, Infliximab, Rituximab, Trastuzumab, Other Types); Indication (Oncology, Autoimmune Diseases, Other Indications); End-User (Hospitals, Cancer Treatment Centers, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Adalimumab segment, which is expected to reach US$16.1 Billion by 2030 with a CAGR of a 23.7%. The Bevacizumab segment is also set to grow at 18.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3.7 Billion in 2024, and China, forecasted to grow at an impressive 19.9% CAGR to reach $6.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Biosimilar Monoclonal Antibody Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Biosimilar Monoclonal Antibody Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Biosimilar Monoclonal Antibody Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, 4D Biomaterials, Bioretec Ltd, Boston Scientific Corporation, CONMED Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Biosimilar Monoclonal Antibody market report include:
- Amgen Inc.
- Biocon Biologics Ltd.
- Biogen Inc.
- Boehringer Ingelheim GmbH
- Celltrion Healthcare Co., Ltd.
- Dr. Reddy's Laboratories Ltd.
- Fresenius Kabi AG
- Henlius Biotech, Inc.
- Merck & Co., Inc.
- Mylan N.V. (now part of Viatris)
- Novartis AG
- Pfizer Inc.
- Samsung Bioepis Co., Ltd.
- Sandoz International GmbH
- Teva Pharmaceutical Industries Ltd.
- Alvotech
- Apobiologix (Apotex Inc.)
- Coherus BioSciences, Inc.
- Formycon AG
- Xbrane Biopharma AB
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Amgen Inc.
- Biocon Biologics Ltd.
- Biogen Inc.
- Boehringer Ingelheim GmbH
- Celltrion Healthcare Co., Ltd.
- Dr. Reddy's Laboratories Ltd.
- Fresenius Kabi AG
- Henlius Biotech, Inc.
- Merck & Co., Inc.
- Mylan N.V. (now part of Viatris)
- Novartis AG
- Pfizer Inc.
- Samsung Bioepis Co., Ltd.
- Sandoz International GmbH
- Teva Pharmaceutical Industries Ltd.
- Alvotech
- Apobiologix (Apotex Inc.)
- Coherus BioSciences, Inc.
- Formycon AG
- Xbrane Biopharma AB
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 235 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
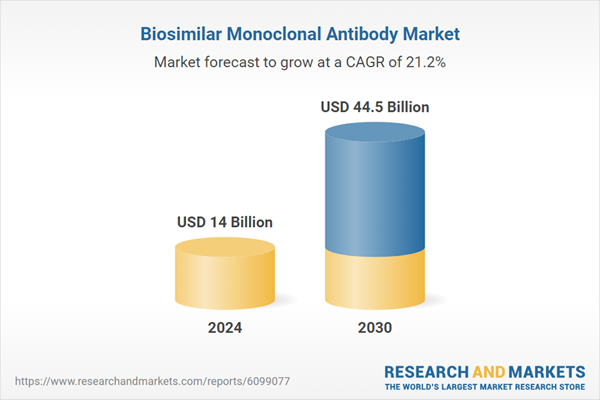
| Estimated Market Value ( USD | $ 14 Billion |
| Forecasted Market Value ( USD | $ 44.5 Billion |
| Compound Annual Growth Rate | 21.2% |
| Regions Covered | Global |