Global Catheter-Related Bloodstream Infections (CRBSIs) Market - Key Trends & Drivers Summarized
Why Are Catheter-Related Bloodstream Infections a Persistent Challenge in Critical Care and Hospital Infection Control?
Catheter-related bloodstream infections (CRBSIs) remain a significant cause of morbidity, mortality, and increased healthcare costs in clinical settings - particularly in intensive care units, oncology wards, and long-term care facilities. These infections arise when pathogens colonize intravascular catheters, leading to systemic bloodstream infections that complicate treatment outcomes and extend hospital stays. As central venous catheters (CVCs) are widely used for medication administration, parenteral nutrition, and hemodynamic monitoring, CRBSIs represent a serious nosocomial risk that continues to strain hospital infection control protocols.Despite improved awareness, the global burden of CRBSIs persists due to inconsistent adherence to aseptic insertion techniques, prolonged catheter dwell times, and antimicrobial resistance. The need to minimize preventable infections is intensifying as healthcare systems prioritize quality metrics, patient safety, and cost containment - driving sustained demand for advanced prevention tools, diagnostics, and bundled care solutions targeting CRBSIs.
How Are Innovations in Catheter Materials, Antimicrobial Coatings, and Infection Surveillance Improving CRBSI Prevention?
Medical device manufacturers are advancing catheter technologies through the use of antimicrobial and antiseptic coatings (e.g., silver sulfadiazine, chlorhexidine, and minocycline-rifampin) that inhibit microbial adhesion and biofilm formation. These coated catheters are increasingly adopted in high-risk settings to reduce infection rates without compromising vascular access functionality. Improvements in catheter insertion kits, securement devices, and transparent dressings are further enhancing infection barrier protection.Electronic health record (EHR)-linked infection surveillance systems and real-time analytics platforms are also supporting early detection and tracking of CRBSIs. Standardized central line bundles, clinical decision support tools, and nurse-driven removal protocols are being implemented to drive compliance and accountability in line care. Additionally, point-of-care diagnostic tools and rapid culture systems are aiding timely pathogen identification and targeted antibiotic administration, improving patient outcomes.
Where Is Demand for CRBSI Prevention and Management Solutions Growing and Which Clinical Environments Are Driving Adoption?
North America leads global CRBSI prevention efforts, supported by well-established hospital quality reporting frameworks and stringent CMS and CDC guidelines. The U.S. continues to drive demand for coated catheters, diagnostic tests, and infection surveillance software. Europe follows with increasing adoption of catheter care bundles and region-wide patient safety initiatives. Asia-Pacific is witnessing strong growth in infection control markets, particularly in countries like India, China, and Japan, where expanding ICU infrastructure and rising surgical volumes are heightening CRBSI prevention priorities.Key clinical environments include intensive care units, oncology departments, dialysis centers, and long-term acute care hospitals - settings where central venous access is common and patient vulnerability is high. Additionally, home infusion therapy and ambulatory care services are expanding the scope of CRBSI risk management beyond hospital walls, driving demand for portable infection prevention solutions and patient education tools.
What Is Fueling the Global Growth of the Catheter-Related Bloodstream Infections Market?
The global CRBSIs market is being driven by the rising use of vascular access devices, growing awareness of hospital-acquired infections (HAIs), and regulatory pressure to meet infection control benchmarks. As healthcare providers focus on reducing preventable harm and optimizing clinical outcomes, investment is increasing in bundled solutions that combine coated catheters, securement systems, and staff training protocols. The financial burden of CRBSIs - through increased treatment costs, penalties, and reputational risk - continues to reinforce the strategic imperative for effective prevention.Innovation in catheter materials, AI-enabled infection tracking, and targeted antimicrobial therapies is accelerating product development and adoption. Strategic collaborations between device manufacturers, infection control teams, and health IT vendors are enabling more integrated, data-driven CRBSI mitigation strategies. As global health systems strive to eliminate catheter-associated complications, a defining question frames the path forward: Can CRBSI prevention evolve into a standardized, evidence-based, and technology-augmented framework - while ensuring cost-effectiveness, scalability, and patient safety across varied care delivery environments?
Report Scope
The report analyzes the Catheter-Related Bloodstream Infections market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Treatment Type (Anti-Microbial Agents, Antibiotic Lock Therapy); Source of Infection (Coagulase-Negative Staphylococcus, S. aureus, Enteric Gram-Negative Bacilli, Yeasts, Enterococci & Streptococci, Pseudomonas, Other Sources of Infection); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Anti-Microbial Agents segment, which is expected to reach US$1.2 Billion by 2030 with a CAGR of a 4.8%. The Antibiotic Lock Therapy segment is also set to grow at 2.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $398.9 Million in 2024, and China, forecasted to grow at an impressive 7.5% CAGR to reach $380 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Catheter-Related Bloodstream Infections Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Catheter-Related Bloodstream Infections Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Catheter-Related Bloodstream Infections Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ACE Cash Express, Advance America, American Express Company, CAN Capital Inc., Capify and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Catheter-Related Bloodstream Infections market report include:
- 3M Company
- AngioDynamics Inc.
- B. Braun Melsungen AG
- Becton, Dickinson and Company
- Biomerics LLC
- Boston Scientific Corporation
- Cook Medical LLC
- ICU Medical Inc.
- IntraSpecialist Pte Ltd.
- Medcomp
- Medline Industries LP
- Medtronic plc
- Merit Medical Systems Inc.
- Navilyst Medical Inc.
- Nexus Medical LLC
- Poly Medicure Ltd.
- Pursuit Vascular (acquired by ICU Medical)
- Smiths Medical (now part of ICU Medical)
- Teleflex Incorporated
- Vygon SA
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3M Company
- AngioDynamics Inc.
- B. Braun Melsungen AG
- Becton, Dickinson and Company
- Biomerics LLC
- Boston Scientific Corporation
- Cook Medical LLC
- ICU Medical Inc.
- IntraSpecialist Pte Ltd.
- Medcomp
- Medline Industries LP
- Medtronic plc
- Merit Medical Systems Inc.
- Navilyst Medical Inc.
- Nexus Medical LLC
- Poly Medicure Ltd.
- Pursuit Vascular (acquired by ICU Medical)
- Smiths Medical (now part of ICU Medical)
- Teleflex Incorporated
- Vygon SA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 384 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
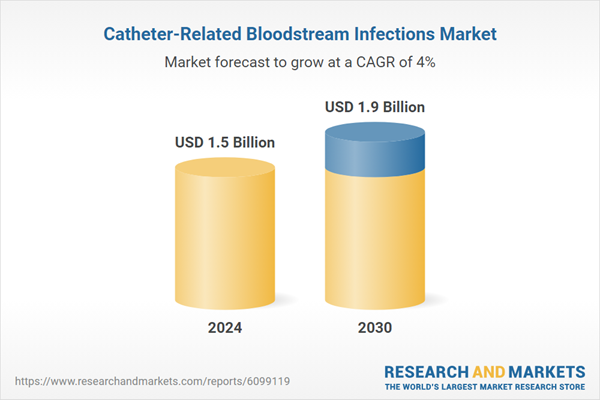
| Estimated Market Value ( USD | $ 1.5 Billion |
| Forecasted Market Value ( USD | $ 1.9 Billion |
| Compound Annual Growth Rate | 4.0% |
| Regions Covered | Global |